Abstract
Aims: There are limited data regarding clinical outcomes of drug-eluting stents (DES) in saphenous vein grafts (SVGs) compared to bare metal stents (BMS). Here we compared outcomes of DES in de novo SVG lesions versus BMS in contemporary percutaneous coronary intervention (PCI).
Methods and results: We compared in-hospital, 6-month, 1-year and two years outcomes in 68 patients (72 grafts) who underwent PCI of SVG lesions using DES and a control BMS group composed of 43 patients (46 grafts) who underwent angioplasty in de novo SVG lesions. Major adverse cardiac events (MACE) included death, myocardial infarction (MI), target lesion revascularisation (TLR), and target vessel revascularisation (TVR). The rates of TLR and TVR at the 1-year evaluation were lower in the DES group than the BMS group (TLR per patient, 7.4% vs. 21%, P=0.04; TVR per patient, 10.3% vs. 23.3%, P=0.1). MACE-free survival was 88.2% in the DES group and 69.8% in the BMS group (P=0.02). At two years clinical follow-up: death 2.9% vs. 4.7% (P=0.6); MI: 8.8% vs. 7% (P=0.6). The rates of TLR and TVR were significantly lower in the DES group compared to the BMS group (TLR per patient, 14.7% vs. 32.6%, P=0.03; TVR per patient, 10.3% vs. 27.9%, P=0.02). The rate of MACE-free survival was 79.4% in the DES group and 58.1% in the BMS group (P=0.02). Between one to two years after PCI, no cases of angiographic stent thrombosis were recorded in either group.
Conclusions: DES implantation in SVG lesions was safe and had favourable outcomes after two years without excess cardiac mortality.
Introduction
Percutaneous revascularisation of obstructive atherosclerotic disease in saphenous vein graft (SVG) lesions remains one of the great challenges in cardiovascular medicine. At present, percutaneous coronary intervention (PCI) is the preferred method for treating patients with SVG lesions, due to the significantly higher risk inherent in second intervention with repeated coronary artery bypass graft.1-2 Compared to balloon angioplasty, bare metal stent (BMS) implantation in SVG lesions has been shown to improve procedural outcomes and reduce major cardiac events. However, no significant reduction in binary restenosis rate was confirmed, and it remains as high as 20-37%.3-4
Recently, drug-eluting stents (DES), either sirolimus-eluting stents (SES) (Cypher, Cordis Corp., Warren, NJ, USA) or paclitaxel-eluting stents (Taxus, Boston Scientific, Natick, MA, USA), have had a significant impact on the restenosis process in selected de novo and restenotic native coronary artery lesions.5-8 However, data regarding the treatment of SVG lesions with DES are limited. While the initial registry reports are encouraging but inconsistent9-14 a recent secondary post hoc analysis, reported increased long-term mortality using DES (sirolimus-eluting stents) for SVG disease as compared to BMS15. The problems of greater local prothrombotic conditions in the SVG and delays in endothelial healing after DES placement are possible drawbacks of DES implantation in SVGs, as they may lead to a greater risk of acute, subacute, and late thromboses. In addition, the mechanism of in-stent restenosis in SVGs may be prolonged and differ from native coronary artery lesions, which may further reduce DES efficacy in reducing angiographic late loss.16-19 We report the 2-year clinical results of patients undergoing DES implantation in SVG lesions as compared to BMS implantation to determine he efficacy and safety of DES use in SVG de novo lesions.
Methods
The study was approved by the IRB committee. All patients gave informed, written consent prior to the catheterisation procedure.
The study is a retrospective analysis of patients who underwent PCI of de novo SVG lesions. We identified 68 consecutive patients (72 grafts) who underwent PCI in de novo SVG lesions using DES (Cypher, Cordis Corp. or Taxus, Boston Scientific, Natick, MA, USA) from January 2004 to December 2005. A BMS control group was composed of 43 consecutive patients (46 grafts) who underwent percutaneous treatment in SVG de novo lesions with BMS during the 12 months immediately prior to the introduction of DES in our institution.
All patients were referred for PCI based on clinical symptoms or provocable ischaemia documented with noninvasive imaging. Patients were pretreated with aspirin and clopidogrel. A 300-600 mg loading dose of clopidogrel was administered prior to the index procedure in patients who were not pretreated. Anticoagulation treatment with unfractionated heparin or bivalirudin was given prior to PCI. Platelet glycoprotein IIb/IIIa receptor inhibitors and distal protection devices were used at the discretion of the operator. PCI was performed in patients with stenotic lesions (> 70% diameter) that involved an SVG, using standard techniques and a femoral approach. Selection of DES or BMS and predilatation with undersized balloons was left to the operator’s discretion. The unavailability of DES larger than 4.0 mm diameter was a common reason for selecting BMS, even after DES became available (patients treated with BMS in the DES era were not included in the analysis). The diameter of a stent could be enlarged by post-dilating the stent with a larger angioplasty balloon to leave a minimal residual stenosis and optimise stent apposition to the vessel wall. All stents were implanted with moderate to high deployment pressure (10-14 atm). Patients were prescribed lifelong aspirin and clopidogrel for at least six months after DES implantation and at least one month after BMS implantation. Patients were excluded from the study if admitted with cardiogenic shock, had an acute myocardial infarction (MI) <24 hours before the index procedure or implantation of a covered stent or a history of brachytherapy or restenotic lesion. Patients with contraindications to aspirin, or clopidogrel or unsuccessful PCI (five patients) were excluded from the current analysis.
The patient registry includes detailed demographic, clinical, angiographic, and procedural data. Immediate and in-hospital events were recorded, and each patient completed a standardised questionnaire either by telephone or in the outpatient clinic at 1-month, 6-month, 12-months and 2-years follow-ups. Survival status at follow-up was assessed by the Interior Ministry registries. Repeat revascularisation procedures and episodes of acute MI were prospectively collected in the hospital database. For patients admitted to peripheral hospitals in the acute phase, the diagnosis of MI was confirmed by documentation of the referring physician. Major adverse cardiac events (MACE) included MI, target lesion revascularisation (TLR), and target vessel revascularisation (TVR). TLR was defined as a repeated revascularisation procedure (either PCI or coronary bypass surgery), as the result of restenosis in the stented segment. TVR was defined as a new revascularisation procedure in the target vessel, also including TLR. Definite stent thrombosis were included defined as an acute coronary ischaemic event associated to angiographic or autopsy documentation of partial or total stent occlusion or thrombosis.
Angiographic analysis
Angiographic films were reviewed at our angiographic core laboratory using the MDViewTM Quantitative Angiographic System (MedconTM Telemedicine Technology, Tel-Aviv, Israel). Analysis was performed by an experienced cardiologist who was unaware of the clinical outcomes. Standard morphologic criteria were used to identify lesion location, lumen diameter and stent length, and thrombus. Using the contrast-filled guiding catheter (i.e., 6 or 7 Fr) as the calibration standard, reference and minimal lumen diameter was determined before and after PCI using an automated edge-detection algorithm. Based on these measurements, percent diameter stenosis was determined before and after intervention, and Thrombolysis In Myocardial Infarction (TIMI) flow grade (0 to 3) was measured prior to, and at the completion of, PCI. Angiographic success was defined as an implant with <50% diameter residual stenosis with TIMI flow grade 3.
Statistical methods
Continuous variables are presented as mean±standard deviation. Chi-square and Fischer’s exact tests were used for analysis of categorical variables when appropriate, and the Student’s t-test was used to analyse continuous variables. Multivariate logistic regression analysis was performed to determine significance of variables related to two years MACE. The model included: diabetes mellitus, renal failure; reference vessel diameter, stent stretching and the use DES. Statistical analysis was performed using STATISTICA software (StatSoft, Inc., Tulsa, OK, USA), and P values <0.05 were considered significant for all analyses.
Results
Baseline characteristics
The clinical characteristics of the two study groups are detailed in Table 1.
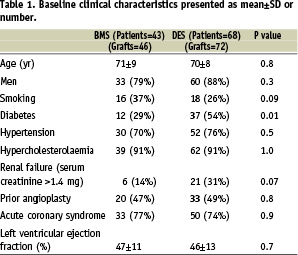
The mean age of the patients was 70±8 years in the DES group and 71±9 years in the BMS group (P=0.8). Acute coronary syndrome was the indication for PCI in 50 DES patients (74%) and 33 BMS (77%) patients (P=0.9). Other baseline characteristics were similar for the two groups, except for a higher incidence of diabetes mellitus in the DES group (54% vs. 29%, P=0.01).
Angiographic and procedural characteristics are presented in Table 2.
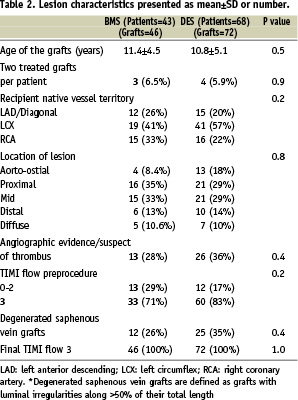
The mean age of SVGs was 10.8±5.1 years in the DES group and 11.4±4.5 years in the BMS group (P=0.5). Cypher stents were implanted in 89% of the patients in the DES group (in 11% the paclitaxel-eluting stents were used).
Angiographic and procedural characteristics (Table 3)
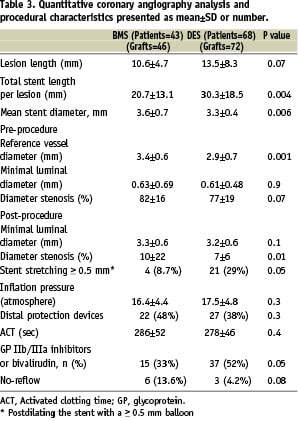
The DES group had longer lesions and longer implanted stents than the BMS group (30.3±18.5 mm vs. 20.7±13.1 mm, P=0.004). The DES group had a smaller mean reference lumen diameter compared to the BMS group (2.9±0.7 mm vs. 3.4±0.6 mm, P= 0.001). The mean minimal luminal diameter was comparable for the DES and BMS groups (P=0.9). The use of glycoprotein IIb/IIIa inhibitors or bivalirudin was 52% in the DES group and 33% in the BMS group (P=0.05). Distal protection devices were used in 38% of the DES cases and 48% of the BMS cases. Procedural success was achieved for all grafts. Angiographs taken immediately after PCI demonstrated similar minimal lumen diameter in the DES (3.2±0.0.6 mm) and BMS (3.3±0.6 mm) groups (P=0.1). The percent diameter stenosis was 7±6% in the DES group compared to 10±22% in the BMS group (P=0.001).
Clinical follow-up (Table 4)
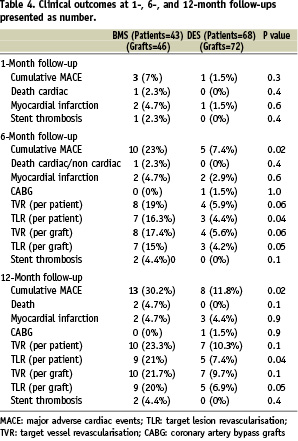
Complete information was available for all of patients at follow-up. There were no significant differences between the two groups at one month: MACE in the BMS group (7%) than the DES group (1.5%, P=0.3), MI in two patients (4.7%) of the BMS patients and one (1.5%) of the DES patients (P=0.6). One of the BMS patients with severe left ventricular dysfunction suffered fatal MI.
All surviving patients participated in the 6-month follow-up. Cumulative MACE at the 6-month follow-up was 7.4% in the DES group and 23% in the BMS group (P=0.02). Between the end of the first month and six months after treatment, no further death or MI events were recorded in the BMS group. One DES patient suffered a nonfatal MI. The rates of TLR and TVR (all ischaemia-driven PCI performed because of anginal complains or evidence of myocardial ischaemia during exercise or pharmacological stress test) were significantly lower in the DES group compared to the BMS group (TLR per patient, 4.4% vs. 16.3%, P=0.04; TVR per patient, 5.9% vs. 19%, P=0.06). The MACE-free survival rate was 92.6% in the DES group and 77% in the BMS group (P=0.02).
All surviving patients participated in the 1-year clinical follow-up. The cumulative MACE at the 1-year follow-up was 11.8% in the DES group and 30.2% in the BMS group (P=0.02). Between 30 days to one year after PCI, no cases of angiographic stent thrombosis were recorded in either group. No additional mortality in both groups occurred. One additional case of nonfatal MI occurred in a DES patient. The rates of TLR and TVR (all ischaemia-driven PCI) were significantly lower in the DES group compared to the BMS group (TLR per patient, 7.4% vs. 21%, P=0.04; TVR per patient, 10.3% vs. 23.3%, P=0.1). The rate of MACE-free survival was 88.2% in the DES group and 69.8% in the BMS group (P=0.02).
Table 5 shows the overall rate of events that occurred in the two groups at 18 and 24 months clinical follow-up.
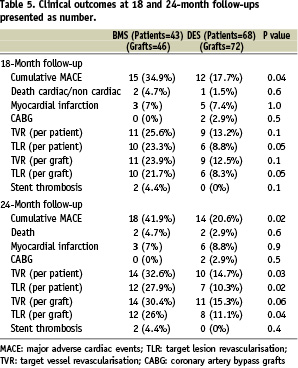
Between one and two years, two patients (2/68=2.9%) in the DES group died compared to no additional death in the BMS group (P=0.4). One patient died from ventricular fibrillation after 13 months of stenting while on dual antiplatelet therapy, and the second suffered sudden death after 18 months of stenting three months after clopidogrel was stopped. The possibility of possible stent thrombosis according to the ARC criteria20 can not be excluded at least in the second case.
Clinical follow-up at two years is shown in Figure 1.
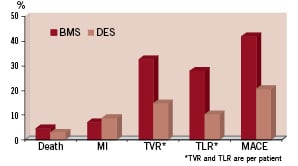
Figure 1. Clinical driven events at two years.
Myocardial infarction occurred in 8.8% in a DES patient versus 7% in the BMS group (P=0.6). The rates of TLR and TVR (all ischaemia-driven PCI) were significantly lower in the DES group compared to the BMS group (TLR per patient, 14.7% vs. 32.6%, P=0.03; TVR per patient, 10.3% vs. 27.9%, P=0.02). The rate of MACE-free survival was 79.4% in the DES group and 58.1% in the BMS group (P=0.02). Between one to two years after PCI, no cases of angiographic stent thrombosis were recorded in either group.
Multiple logistic regression analysis adjusted for diabetes mellitus, renal failure; reference vessel diameter, stent stretching and the use DES, showed that the use of DES is an independent predictors for two years MACE (odds ratio 0.4; 95% confidence interval, 0.14-.96; P=0.04).
To overcome the possible differences between the two DES stents (Cypher, Cordis Corp., Miami Lakes, FL, USA, or Taxus, Boston Scientific, Natick, MA, USA) we performed a separate analysis for the Cypher stents patients (Table 6).
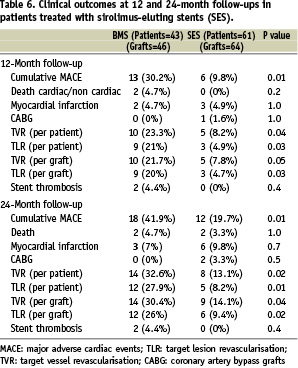
This analysis did not make any significant changes in clinical outcomes suggesting minor effects of the small number of patients treated with the Taxus stent.
Discussion
We found that the use of DES in de novo SVG lesions was safe and effective in reducing the incidence of TLR. DES treatment improved MACE-free survival without increasing thrombotic complications up to two years after PCI. Our DES results were excellent despite an aged DES cohort (70±8 years) with reduced left ventricular function (mean ejection fraction 46±13%) and a relatively high percentage of acute coronary syndromes (74%) and diabetes mellitus (54%) on admission. DES treatment in SVG was effective and thrombotic resistant for two years.
Unlike native coronary arteries, SVGs are large vessels with diffuse, concentric and friable plaque burdens with a thin or absent fibrous cap and abundant lipid debris that is in contact with the blood.17 Furthermore, the mechanism of in-stent restenosis in stented SVGs may differ from that in native coronary arteries. A combination of intimal hyperplasia, progression of atherosclerosis, local inflammatory reaction to stent material, and thrombosis appears responsible for SVG restenosis.16-18 Those conditions made the safety and efficacy of DES in SVGs uncertain. Indeed, recently published data from the Prospective Multicentre German Cypher Stent Registry21 showed that percutaneous treatment of SVGs predicted MACE and TVR at follow-up in the DES era. Of a total of 1,977 lesions in 1,726 patients treated with DES, 132 lesions were located in an SVG. The TVR rate was 21.2% in this lesion subset. Chu et al14 found no increase in event-free survival one year after DES were used (48 patients, 50 SVG lesions) compared to BMS (57 patients, 64 SVG lesions). Their data suggested that treating SVGs remains a therapeutic challenge despite the use of DES.
Our findings, and those of others, are in accordance with a recent randomised trial comparing sirolimus-eluting stents to BMS,22 which demonstrated that DES are effective in reducing target lesion revascularisation in SVGs. Compared to previous studies,9-14 the present study included patients with more challenging lesion characteristics, including 74% with acute coronary syndromes, 54% with diabetes mellitus, and 31% with renal failure. These subgroups are known to be associated with less favourable outcomes even when using DES.10,11,17 Previous studies have demonstrated favourable results lasting up to nine months with DES, and the present study extents those results to two years in all patients. Previous studies that evaluated patients up to six months10 or had a small sample number (19 patients with 22 SVG lesions),11 demonstrated that the use of DES (either with paclitaxel or sirolimus) was safe and feasible and lead to a reduction in the incidence of restenosis and a consequent increase in MACE-free survival. No events related to acute, subacute, or late stent thrombosis were reported.10 In the RESEARCH registry,11 at one year, the incidence of MACE was 16%, with only one (5%) incidence of TLR. Price et al12 published a retrospective report that included 35 patients who underwent DES placement in 39 de novo SVG lesions. The TLR rate was 6%, and the overall MACE rate at the 7-month follow-up was 20%. Our study, which included a larger number of patients and extended follow-up, showed favourable results of DES implantation without any increase in thrombotic events. Unlike a recent randomised trial22, we did not exclude patients with recent acute MI, reduced left ventricular function, or renal failure. We also included patients with more severe diameter stenosis (> 70% compared to >50% diameter stenosis) and total occlusion. These features make our study more representatives of the most compromised patients, and their outcomes were different than those of participants in randomised controlled trials.23
With recent safety concerns regarding long-term adverse outcomes for the “off-label” DES use it is assuring that unlike the recent secondary post hoc analysis reporting excess risk of late death after sirolimus-eluting stent (SES) placement in patients with diseased SVG (all within two years after stenting)15 we did not find such an increased mortality. It is obvious that more scientifically rigorous data on this important subset of patients is needed.
Study limitations
Our study has several important limitations. For instance, it is a single-centre, historical retrospective, non-randomised study that was subject to bias in patient selection, technique, and operators skills. It is possible that our skills in PCI of SVG improved with time, thus the good results seen with DES are not only explained by the device per se, but also by the higher expertise of the operators. Furthermore, the majority of patients in the BMS group were treated prior to DES becoming available at our institution, which may have contributed to selection bias. Second; cardiac enzymes were not evaluated after each procedure to document peri-procedural complications, such as MI. Since routine cardiac enzyme assessment was not performed, it is possible that ‘’enzyme MI,’’ without apparent ECG changes or symptoms, may have been overlooked, thereby underestimating the incidence of non-Q wave MI. Additionally, we did not perform systematic angiographic follow-ups. However, patients who did not undergo repeat angiography were clinically well at the follow-up evaluations. Follow-up angiography was clinically driven, and TVR was performed at follow-up in both groups based on the presence of anginal symptoms or abnormal stress testing. Another limitation could be related to the differences between the reference vessel diameter, which was smaller in the DES group. This difference decreased after stenting and oversizing the stent in the DES group. There is a possibility that oversizing the DES by stretching may influence the outcome due to less stent/vessel wall area. This cannot be excluded, although previous reports24 showed that implantation of a 3.0 mm DES with post-dilation with a 3.5-4 mm balloon did not result in any significant difference in complications, non-Q-wave MI, or TLR. Finally, the recommended duration of double antiplatelet therapy was for at least six months after DES implantation. Recent recommendations suggest that double antiplatelet therapy should be recommended in all patients receiving drug-eluting stents for at least 12 months25. It can’t be excluded that longer duration of dual antiplatelet therapy in both groups affect our results. Despite these limitations, our findings represent a large cohort of patients treated for SVG disease with DES implantation and complete clinical follow-up. We found that DES implantation in SVG lesions was safe with favourable and improved outcomes after one year.
Conclusions
Percutaneous revascularisation in SVG lesions with DES appears safe with a high procedural success rate. Compared to BMS implantation, DES implantation in SVG de novo lesions is associated with a reduction in the TLR rate and a beneficial effect on MACE-free survival after two years without excess mortality.

