Abstract
Aims: The main objective was to use IVUS-backscatter radiofrequency (IVUS-RF) to assess the degradation of a bioabsorbable stent by measuring serial changes in dense calcium (DC) and necrotic core (NC) as assessed by intravascular ultrasound-Virtual Histology™ (IVUS-VH) and in the strain as assessed by palpography.
Methods and results: In the ABSORB trial, 27 patients treated with a single bioabsorbable everolimus-eluting stent (BVS, Abbott Vascular, Santa Clara, CA, USA) were all imaged with IVUS-RF post-stenting and at 6-month follow-up, and 13 and 12 patients were also investigated pre-stenting with IVUS-VH and palpography respectively.
From pre- to post-stenting, with VH (n=13), there was an increase in mean “DC” (9.8 vs. 25.4%, p=0.0002) and “NC” (15.5 vs. 30.5%, p=0.0002). In palpography (n=12), the mean number of frames with Rotterdam Classification (ROC) III/IV per cm decreased from 1.22±1.91 to 0.12±0.31 (p= 0.0781) and the mean cumulative strain values (all frames with ROC I-IV scores) changed from 0.50±0.27 to 0.20±0.10% (p= 0.0034).
Comparing post-stenting with follow-up (n=27), VH showed a decrease in “DC” (29.7% vs. 21.1%, p=0.0001). “NC” also decreased (26.9 vs. 21.5%, p=0.0027). For palpography (n=25 patients), an increase in the mean number of frames with ROC III/IV per cm was observed from 0.09±0.26 to 0.22±0.36 (p=0.1563) while the mean cumulative strain values (all frames with ROC I-IV scores) changed from 0.15±0.10 to 0.26±0.12% (p<0.0001).
Conclusions: IVUS-VH changes at 6 months suggest alteration of the BVS with reduction of RF backscattering by polymeric struts. Strained plaques on the palpograms were almost abolished following stent implantation. However, strain values reappeared within 6 months suggesting an increase in endoluminal deformability of the stented vessel.
Introduction
Metal stent use has been one of the major breakthroughs in the treatment of patients with coronary artery disease.1 However, the many advantages are somewhat counterbalanced by associated pitfalls such as restenosis, the persistent metal presence that may preclude late surgical revascularisation of the treated vessel, and jailing of side branches.2-9 The advent of a new bioabsorbable everolimus-eluting stent10 promises to overcome some of these disadvantages. The in vivo serial changes in its structural conformation (polymer degradation profile) were established in a porcine coronary artery model. Mass loss was approximately 30% at 12 months with a further reduction to 60% mass loss by 18 months after implantation10. However, in human coronary arteries the bio-absorption process has never been explored. Hence in the ABSORB trial all clinically available and potentially useful invasive coronary imaging techniques to assess bio-absorption of polymeric struts were used (i.e. echogenicity, intravascular ultrasound [IVUS] – radiofrequency [RF] data analysis: Virtual Histology™ [VH] and palpography and optical coherence tomography). Nevertheless, none of these techniques have been specifically designed to assess bio-absorption. Specifically, IVUS-VH11,12 is a tool developed to assess the tissue composition of intact native coronary arteries. The analysis of the actual stent area and its surroundings by IVUS-VH identified the stent struts as “dense calcium” and “necrotic core” (white and red colour in the VH colour-code), which correspond to a specific backscattered radiofrequency of the polymeric strut structures (highly echogenic). Palpography is an IVUS-based technique that assesses the local mechanical deformation of the vessel wall by measuring the relative alteration of backscattered radiofrequency signals at two different blood pressure levels. For instance, lipid-rich vulnerable plaques have greater deformation and higher strain values compared to calcific or fibrous plaques.13 The main objective was twofold: i) to use IVUS-VH to follow-up the degradation of a bio-absorbable everolimus-eluting stent by measuring the temporal changes in IVUS-VH characteristics at pre-, post-stenting and at 6 months; and ii) to evaluate the temporal changes in palpographic strain values in the stented segment.
Methods
Study design
The study design for the prospective, open label ABSORB trial has already been published elsewhere.10 Briefly, this was a single arm study that enrolled 30 patients at 4 participating sites between March and July 2006. Patients were older than 18 years, with a diagnosis of stable, unstable or silent ischaemia. All treated lesions were single, de novo in a native coronary artery of 3.0 mm, shorter than 8 mm for the 12 mm stent or <14 mm for the 18 mm stent (only two patients received an 18 mm stent), with a % diameter stenosis >50% and <100% and a thrombolysis in myocardial infarction (TIMI) flow grade of >1. Major exclusion criteria were patients presenting with an acute myocardial infarction, unstable arrhythmias or patients who had left ventricular ejection fraction <30%, restenotic lesions, lesions located in the left main coronary artery, lesions involving a side branch >2 mm in diameter, and the presence of thrombus or another clinically significant stenosis in the target vessel.
The study was approved by the ethics committee at each participating institution and each patient gave written informed consent before inclusion.
Study device
The BVS stent has a polymer backbone of Poly-L (racemic)-lactic Acid (PLLA) coated with a Poly-D (racemic), L-lactic acid (PDLLA) polymer that contains and controls the release of the anti-proliferative drug everolimus. Both PLLA and PDLLA are fully absorbable. The absorption process occurs via hydrolysis: the long chains of PLLA/PDLLA become shorter as bonds between the repeating units are hydrolysed, producing lactic acid which is metabolised via the Krebs cycle, and small particles <2 microns diameter that are phagocytosed by macrophages. The time for complete absorption of the polymer backbone is predicted from preclinical studies to be about 2-3 years whereas the polymer coating is absorbed in approximately 9 months.
Stenting procedure
Lesions were treated with routine interventional techniques that included mandatory pre-dilatation using a balloon shorter than the study device and 0.5 mm less in diameter. The BVS stent was implanted at a pressure not exceeding the rated burst pressure (16 atm). All patients were pretreated with aspirin and a loading dose of at least 300 mg of clopidogrel was administered according to local hospital practice. After the procedure, all patients were to receive aspirin >75 mg daily for the study duration (5 years) and clopidogrel 75 mg daily for a minimum of 6 months. Anticoagulation and glycoprotein IIb/IIIa inhibitor use was according to local hospital practice.
Imaging procedure
Pre-stenting IVUS-VH (n=13) and pre-stenting palpography (n=12) imaging were obtained in a subgroup of patients treated at the Thoraxcenter, Erasmus MC, Rotterdam, The Netherlands. The purpose of these analyses was to observe the acute changes after the implantation of the BVS stent. Subsequently, in the entire population IVUS-RF was obtained post-stenting and at follow-up. Both imaging techniques were acquired simultaneously with a phased array 20 MHz intravascular ultrasound catheter (EagleEye™; Volcano Corporation, Rancho Cordova, CA, USA) using automated pullback at 0.5 mm per second. Four tissue components (necrotic core – red; dense calcium – white; fibrous – green; and fibro-fatty – light green) were identified with autoregressive classification systems.11,14 Each individual tissue component was quantified and colour coded in all IVUS cross sections as previously described. In a previous post-mortem validation study, RF analysis demonstrated sensitivity and specificity for detection of necrotic core of 92% and 97%, respectively.14 All IVUS- VH analyses were performed offline using pcVH 2.1 (Volcano Corporation, Rancho Cordova, CA, USA) by an independent clinical research organisation (Cardialysis, Rotterdam, The Netherlands). For each cross section, polymeric stent struts were detected as areas of apparent “dense calcium” and “necrotic core”. We used the change in quantitative analyses of these characteristics between implantation and follow-up as a surrogate assessment of the polymer bio-absorption process.
IVUS-based palpography assesses deformability of the plaque. The underlying principle is that softer tissue is more readily deformed compared with harder tissue when force (eg, pulsatile arterial pressure) is applied.13 The deformability of coronary plaque is quantified using the analysis of radiofrequency signals at different diastolic pressure levels. The strain is normalised to a pressure difference of 2.5 mm Hg per frame. This allows the construction of a ‘strain’ image in which harder (low strain) and softer (high strain) regions of the coronary arteries can be identified, with radial strain values ranging between 0% and 2%.13 In post-mortem coronary arteries that were investigated with histology and IVUS palpography, the sensitivity and specificity of palpography to detect high strain values were 88% and 89%, respectively.13
Plaque strain values were assigned a Rotterdam Classification (ROC) score ranging from I to IV (ROC I: 0% to 0.6%; ROC II: 0.6% to <0.9%; ROC III: 0.9% to <1.2%; ROC IV: >1.2%) as previously described.15 All IVUS- palpography analyses were performed by an independent clinical research organisation (Cardialysis, Rotterdam, The Netherlands).
The clinical and geometrical analysis in the ABSORB study has already been reported.10 Thus, in this paper, we exclusively report changes in tissue composition and plaque deformability.
Statistical analysis
As stated in the main ABSORB Study, the sample size was not defined on the basis of an endpoint hypothesis but rather to provide information about device efficacy and safety. Therefore, the present substudy should be seen as hypothesis-generating.
Discrete variables are presented as counts and percentages. Continuous variables are presented as means ± SD, quartiles and ranges. A two-sided p-value of less than 0.05 indicated statistical significance. Due to the exploratory nature of these analyses, p values were unadjusted for multiple comparisons in this manuscript.
The density of high-strain spots per 10 mm was defined as the number of cross-sections with strain values >0.9% (i.e. ROC III or IV) divided by the number of all analysable cross sections in the region of interest and normalised for 10 mm.
Paired comparisons between pre-procedure, post-procedure and follow-up were done by the Wilcoxon signed rank test. Statistical analyses were performed with use of SAS 9.1.
Results
Overall, 30 patients were included in the ABSORB study. The mean age was 62±9 years, most being male patients (60.0%), while 70.0% of the patients presented with stable angina, 26.7% and 3.3% had unstable angina and silent ischaemia respectively. The studied vessel was the left anterior descending in 46.7%, the left circumflex in 30.0% and the right coronary artery in 23.3% of the patients.
Intravascular ultrasound Virtual Histology™
Changes between pre- and post-stenting
In 13 patients, IVUS-VH was performed pre- and post-stenting (Table 1 and Figure 1). The mean percentage of “dense calcium” increased from 9.8% to 25.4% (p=0.0002) and “necrotic core” went from 15.5% to 30.5% (p=0.0002). Fibrous tissue, the major component of the vessel wall when expressed as mean area did not demonstrate statistically significant changes post-stenting, however its relative contribution to the stented vessel wall decreased significantly (60.8% vs. 39.0%, p=0.0002). In a similar fashion the percentage of fibro-fatty tissue decreased (13.9% vs. 5.1%, p=0.0002).
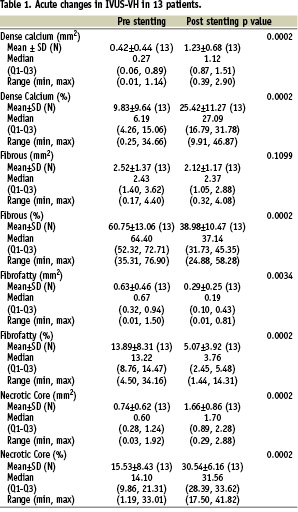
Figure 1. Serial changes in the stent struts as assessed by IVUS Virtual Histology™ (VH). On the left hand side, greyscale IVUS images are shown at pre-, post-stenting and follow-up. In the central part, their corresponding colour code VH images are depicted. On the right hand side, per-cross section quantification in absolute and relative terms is shown. Of note, increases in “dense calcium” (white) and “necrotic core” (red) are noted after stenting. At follow-up, a change in the stent strut appearance in greyscale IVUS is noted and decreases in the “dense calcium” and “necrotic core” contents in IVUS- VH are detected. Fibrous - green; and fibro-fatty - light green.
Changes from post-stenting to follow-up
Overall, in patients (n=27) with post-stenting and follow-up VH, a significant decrease in “dense calcium” (29.7% vs. 21.1%, p=0.0001) was shown. In 21 out of 27 patients, there was a regression in the “calcified” pattern (Table 2 and Figure 2). The content of “necrotic core” also decreased (26.9% vs. 21.5%, p=0.0027). In turn, both fibro-fatty and fibrous tissue increased in terms of both mean areas and percentages (Table 2 and Figure 1).
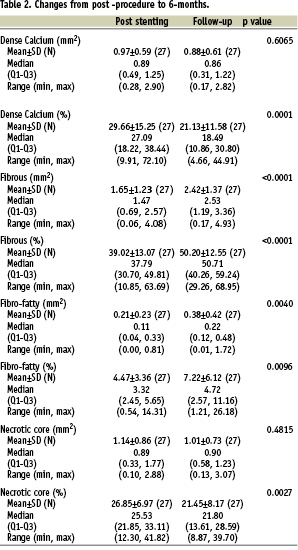
Figure 2. Temporal changes in virtual histology tissue types. From top to bottom, the mean percentage of “dense calcium”, “necrotic core”, fibrous tissue and fibro-fatty tissue is reported. Thirteen patients were imaged at pre-, post-stenting and follow-up (red solid lines). A further 14 patients were only imaged at post-stenting and follow-up (black dotted lines).
Intravascular ultrasound palpography
CHANGES FROM PRE- TO POST-STENTING
In 12 patients, IVUS-palpography was performed pre- and post-stenting (Figure 3 and 4). The mean number of frames with ROC III/IV per cm decreased from 1.22±1.91 to 0.12±0.31 (p=0.0781). The mean cumulative strain values in all frames with ROC I-IV scores changed from 0.50±0.27 to 0.20±0.10% (p= 0.0034).
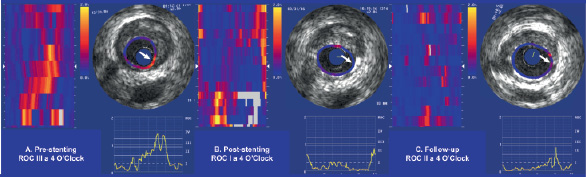
Figure 3. Sequential changes in high-strain values as assessed by IVUS-palpography. Panel A shows an intact plaque with a ROC III spot at 4 o’clock (white arrow). Panel B depicts abolition of this high strain spot following stenting (ROC I). In panel C a slight increase in plaque deformability was documented (ROC II).
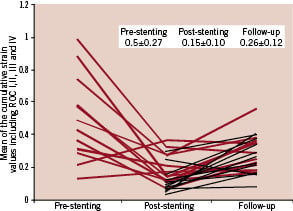
Figure 4. Temporal changes in strain values. The mean cumulative strain values in all frames with ROC I-IV scores are shown. Twelve patients were imaged at pre-, post-stenting and follow-up (red solid lines). Another 13 patients were only imaged at post-stenting and follow-up (black dotted lines). After stenting, abolition of the strain values was noted; however a higher level of plaque deformability was present at follow-up.
CHANGES FROM POST-STENTING TO FOLLOW-UP
In patients (n=25) with post-stenting and follow-up palpography, a slight increase in the mean number frames with ROC III/IV per cm from 0.09±0.26 to 0.22±0.36 (p=0.1563) was observed, while the mean cumulative strain values in all frames with ROC I-IV scores increased significantly from 0.15±0.10 to 0.26±0.12% (p<0.0001) (Figure 3 and 4).
Discussion
In this substudy of the ABSORB clinical trial the main findings in IVUS-VH were an important increase in “dense calcium” and “necrotic core” immediately after stent implantation and a subsequent decrease of these tissue surrogates at 6 months follow-up; this decrease potentially reflects echogenic alteration of the BVS stent struts. On the palpograms, the almost complete initial abolition of the high strain regions was decreased six months following stent implantation: presumably after partial stent absorption the mean strain values increased again.
In a previous study by Tamai et al,16 PLLA stents analysed by greyscale IVUS at 6 months seemed to maintain their scaffolding properties and apparently did not exhibit changes in echogenicity as a sign of biodegradation. In the ABSORB trial, a reduction of the stent area was observed suggesting a mild recoil of the polymeric stent structures.10 Implantation of polymeric struts resulted in the appearance in the vessel wall of highly echogenic structures with radiofrequency backscattering similar to calcific structures (Figure 1). Indeed, in this study using IVUS-VH, an increase in absolute “dense calcium” (Δ0.82 mm2, p=0.0002) from pre- to post-stenting was observed. This dramatic and sudden change in “DC” may be attributed to the introduction of polymeric struts and might correspond to the VH fingerprint of the polymeric struts.
Following BVS stent implantation, the mean fibrous tissue area showed a slight decrease that failed to reach statistical significance (from 2.5 to 2.1 mm2, p=0.1099). The mean fibro-fatty tissue area decreased significantly, although the change is numerically very small, and might be an artefact. From the ultrasonic point of view, the introduction of these highly echogenic structures might affect ultrasound penetration and therefore the backscattering of other tissue components located behind the struts, since potentially less acoustic signals are reaching the tissue.
Although we acknowledge that the classification tree of the IVUS radiofrequency analysis has not been validated for polymeric stent struts, the radiofrequency backscattering is expected to increase with the reduction in the echogenicity of the implanted stent. It is reasonable to assume that the hydrolysis of the polymer which affects its molecular weight and mass, will also alter its acoustic properties.
The BVS stent is constituted of PLLA (backbone) and PDLLA (coating) which are both fully bio-absorbable. During bio-absorption, the long chains of PLLA and PDLLA are progressively shortened and ultimately phagocytosed by macrophages.10 At follow-up, these pseudo-dense calcific structures shrink by nearly 30% (in absolute terms, an 8.6 % reduction in “dense calcium” from 29.7% to 21.1%), which is consistent with the reduction in mass of the BVS polymeric struts observed in animals. These observations are at variance with the previous report by Tamai et al where no signs of biodegradation were observed.16 A possible explanation is that the speed of bioabsorption of a polylactic polymer is greatly influenced by its purity, crystallinity and relative composition of L and D isomers.
The changes in the physical properties of an artery can be described in terms of stiffness, ability to distend and compliance. These characteristics are dependent on the composition of the vascular wall.17 Deployment of a stent against the vessel wall undoubtedly modifies the physical properties of the endoluminal surface. The near-abolition of the high strain values (ROC III/IV) immediately post-stenting reflects major changes in deformability of the plaque and the observed reductions in high strain values may be due to a real decrease in deformability of the scaffolded vessel wall. Alternatively, it may also reflect an ultrasonic artefact, namely the incapability of palpography to measure intrinsic changes in strain of the vessel due to the acoustic properties of the stent struts preventing a proper propagation of radiofrequency signal behind them. The loss of mass observed over time in the porcine model may cause the reappearance of high strain values on the endoluminal surface of the vessel wall. All these observations are indirect indicators of the modifications in strut structure. Although IVUS-VH and palpography have not been validated to detect change in the integrity of polymeric stents, they seem to change acutely and chronically with the introduction and subsequent bio-absorption of the polymeric struts.
These new imaging techniques allow a better understanding of the effect of different therapeutic modalities.
Among the many clinical implications of the use of a biobasorbable stent, the most relevant pertains to the possibility to avoid potentially deadly events such as late stent thrombosis, providing the stent is fully absorbed in a reasonable period of time. Ideally, bio-absorption must occur after neointimal growth has been modulated. Knowing that this occurs mainly during the first 6 months after stent implantation, we have assessed bio-absorption using IVUS radiofrequency data analysis over this period of time. The results of the present substudy are unique findings since the in vivo bio-absorption rate in diseased human arteries is not known.
Limitations
In the context of stent studies, IVUS radiofrequency data analysis faces the following issues: i) misclassification of the stent struts as “dense calcium” and “necrotic core” in VH; ii) with both techniques – VH and palpography – the lack of proper validation to assess polymeric stents and; iii) the potential interference of the superficial stent struts on the backscattering from the tissue behind them. However, these techniques have corroborated other observations of polymer alteration documented with light transmission. In other words, changes in optical coherence of the struts reported elsewhere confirm the ultrasonic modifications reported here.
Conclusions
The quantitative assessment of the IVUS-VH changes at 6 months suggests early strut alteration of the BVS stent with reduction of radiofrequency backscattering. High-strain plaques seen on the palpograms were almost abolished following stent implantation. However, strain values reappeared at 6 months in some patients also suggesting an increase in deformability on the luminal surface of the stented vessel.
Acknowledgements
Authors are grateful to Jurgen Ligthart, Senior IVUS technician in Thoraxcenter, Erasmus MC and Monique Schuijer, ABSORB trial project manager at Cardialysis NL. Authors also thank Susan Veldhof from Abbott Vascular.

