Abstract
Aims: To compare measurements of coronary stent dimensions using a novel, low pressure balloon catheter-based technique - Metricath (MC), with those obtained by intravascular ultrasound (IVUS) and quantitative coronary angiography (QCA).
Background: Intravascular ultrasound (IVUS), the current gold standard to optimize stent placement is expensive, not widely available, and needs expertise for interpretation.
Methods and results: We compared cross-sectional diameter and area measurements obtained by MC, IVUS, and QCA immediately after successful stent implantation. The order of measurements was randomized. Both on-line and off-line (independent core lab) analysis was performed.
Measurements were obtained in 21 patients at 22 stents in the LAD (n=10), RCA (n=6), and LCx (n=6). Nominal stent diameter was 2.5-3.5 mm. Average stent diameter was 2.54±0.28 mm by QCA, 2.77±0.31 mm by IVUS, and 2.86±0.28 mm by MC (P<0.001 QCA versus MC, P=0.13 IVUS versus MC). Results of on-line area measurements showed a small but significant difference between IVUS and MC 0.53 mm2, 95% confidence interval 0.17-0.90 mm2, P<0.01. Regression analysis demonstrated, however, that MC correlated best with off-line IVUS (diameter: y=1.01x, R2=0.83, P<0.001; area: y=1.02x, R2=0.81, P<0.001). Bland-Altman analysis showed a mean difference in diameter between on-line MC and off-line IVUS of 0.03±0.12 mm and between MC and off-line QCA of 0.10±0.23 mm.
Conclusion: MC is a new, promising method providing information on average stent dimensions that is equivalent to that obtained by off-line IVUS analyzed in an independent core lab.
Abbreviations
CRO = clinical research organization
DES = drug-eluting stent
IVUS = intravascular ultrasound
LAD = left anterior descending coronary artery
LCx = left circumflex coronary artery
MC = Metricath
PCI = percutaneous coronary intervention
QCA = quantitative coronary angiography
RCA = right coronary artery
Introduction
The concept that “bigger is better”, namely, that final luminal diameter at the site of percutaneous coronary intervention (PCI) is inversely correlated with the need for reintervention emerged from numerous studies, that used quantitative coronary angiography (QCA), in the era of balloon angioplasty and of bare stent implantation. On-line QCA using edge-detection is often used, in current clinical practice, to guide PCI but has major acknowledged limitations1-5; first, it gives only a 2D outline of the lumen and thus calculated lumen area data is based on assumptions; second, by definition it can only measure lumen and not vessel dimensions thus predisposing to under sizing of balloons/stents. Intravascular ultrasound (IVUS) provides precise measurements of baseline and stented lumen and vessel diameter6,7. However, IVUS is not widely available, adds significantly to the cost of the procedure, and requires considerable expertise for accurate interpretation. While the advent of drug-eluting stents has resulted in a remarkable decrease in restenosis, observational studies suggest that stent underdeployment still plays a major role in in-stent restenosis.
The Metricath System is a novel, low pressure balloon catheter-based technique to measure coronary stent dimensions. That has been validated in a porcine coronary model of stent implantation8. Metricath measurements are obtained in about ten seconds after placement of the catheter and the system provides a direct numerical read-out. We measured coronary stent dimensions using the Metricath (MC) system and compared the results with those obtained by intravascular ultrasound (IVUS) and quantitative coronary angiography (QCA) in patients with coronary artery disease.
Methods
Metricath description
The Metricath system measures both coronary lumen diameter and cross sectional area using a dedicated intracoronary balloon catheter connected to an external console. The balloon is advanced to the target site, using standard interventional techniques, after purging with saline or diluted contrast and calibration. Proximal and distal radio-opaque markers facilitate precise positioning. An external console (Metricath 1000, Fig. 1) inflates the balloon, with sterile fluid, to a maximum (monitored) pressure of 260 mmHg (close to maximum expected systolic blood pressure) then deflates the balloon. Vessel dimensions are derived from measurements of the volume of fluid and the pressure within the balloon.
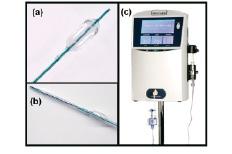
Figure 1a. The Metricath catheter is similar to a conventional PTCA catheter: the measurement balloon (inflated) has a length of 7mm. The catheter shaft contains three lumens: one for balloon fluid infusion, one for measuring balloon fluid pressure, and one for the guide wire. (b) A prototype Metricath catheter contains: both a measurement balloon (inflated) proximal, and a distal angioplasty balloon. The angioplasty balloon requires one additional shaft lumen for conventional inflation. (c) Metricath console: It contains an infusion pump, pressure transducer, LCD display and related hardware.
The “Metricath equation”, which describes the calculations performed by the console software, is given below and explained in Appendix A.
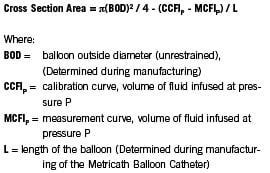
The variables BOD and L are determined for each individual catheter during the “parameterization” stage of manufacture. These variables are stored on a digital information data chip, which is an integral component of each Metricath balloon catheter. The data chip is located at the connector of the pressure transducer of the balloon catheter and it connects to the console. Hence, one only needs the pressure-volume curves obtained during each the ex-vivo calibration cycle (CCFIp) and the in-vivo measurement cycle (MCFIp) to be able to calculate cross sectional area and diameter. The console then displays the diameter and cross-sectional area of the blood vessel at the measurement site. The Metricath System allows for multiple measurements with the same catheter within the same patient. This permits the user to “map” the vascular sections of interest.
Patient selection
Twenty-one patients with coronary artery disease participated in this study. Main inclusion criteria were age between 20 and 80 years old, in whom coronary artery angiography was indicated and angioplasty and stent implantation with a diameter of 2.0 - 3.5mm were being considered and willing to give informed consent in writing. Main exclusion criteria were pregnancy, previous stenting in the target vessel area, a myocardial infarction within 6 weeks prior to the procedure or unstable angina pectoris. Based on diagnostic angiography, patients who did not meet the following exclusion criteria remained eligible for enrolment in the study: -total occlusion of the target vessel; -lesions requiring more than one stent; -visible thrombus, filling defect, or ulceration in the target coronary artery; -severely calcified lesions which suggest that balloon pre-dilation would not achieve adequate luminal diameter to allow successful stent delivery and deployment; -target lesion beyond a left main artery stenosis >50%; -unprotected left main coronary artery; -blood pressure in excess of 180mmHg at the time of the angiography.
The protocol was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, The Netherlands and conducted at the Thoraxcenter and all patients gave written informed consent.
Study design
Following successful stent implantation, randomization by assignment envelopes determined the order in which each respective measurement system was used. No postdilation was allowed between measurements. Clinical data was collected up to patient discharge from hospital, and in addition included a one-month post-discharge telephone call or out-patient clinic visit, for the purpose of monitoring patient safety.
The Metricath System calculates arterial diameter and area over a 7mm segment length (the length of the balloon); therefore, the QCA and IVUS measurements were also averaged over the same 7mm arterial segment. The number of measured segments and their location, as a function of the length of the stent, is presented in Table 1. In addition, average stent dimensions were determined by calculating the mean of the subsegmental data.

QCA Coronary angiograms were obtained using 7F guide catheters in orthogonal views. Quantitative angiographic analyses were performed on-line, and off-line by an independent core lab (Cardialysis; Rotterdam, the Netherlands), using a validated edge detection system (CAAS II, Pie Medical, Maastricht, The Netherlands). Measurements were performed in orthogonal views, where possible, and averaged. Calibration was performed using the known diameter of the guiding catheter as a reference. Mean area and diameter over the length of the stent was recorded. The mean area and diameter was also recorded for each 7mm length segment of the stent, corresponding to the Metricath balloon length.
IVUS was performed using standard techniques with a commercially available 30 MHz mechanical sector scanner (Ultra Cross 2.9, Boston Scientific SCIMED, Maple Grove MN, USA) and an automated pullback device (0.5 mm/sec). Area and diameter measurements were performed on-line using dedicated software. Calibration was provided by the in-built millimeter grid. At least 4-5 images were analyzed for each 7mm segment, while the mean for the stent was obtained by averaging the segment data. Measurements were performed on-line by a catheterization laboratory technician, as well as off-line by an independent core lab (Cardialysis BV, Rotterdam, The Netherlands).
Metricath measurements were taken twice, in succession, without changing the position of the Metricath catheter balloon within the stent. Care was taken to ensure that the edges of the radiopaque markers on the Metricath Balloon Catheter were within the stented section of artery. Measurements were also averaged for the entire stent.
Data management and statistical analysis
Monitoring and data management was performed by an independent CRO (Cardialysis BV, Rotterdam, The Netherlands). Data are expressed as mean ± SD. Agreement between QCA, IVUS and Metricath was analyzed using the Bland and Altman method9. Data are given as plots showing the absolute difference between corresponding measurements against their average. The relative difference between measurements (absolute difference divided by the average) gives the bias, its standard deviation gives the random variation. Absolute data were analyzed for correlation by regression analysis as a necessary, but not sufficient, condition for agreement. Data of all groups were compared by ANOVA, followed by t-test if ANOVA showed significant differences. A p- value less than 0.05 (two tailed) was considered statistically significant. When tests were performed repeatedly, an appropriate lower p-value was considered to indicate statistical significance.
Results
Measurements were performed in 21 patients, age 62±8 years, 12 male. Unstable angina was present in 10 patients. Stents (nominal diameter 2.93±0.26 mm; nominal length 17.1±7.4 mm) were deployed in the left anterior descending (n=10), circumflex (n=6), and right coronary artery (n=6).
The Metricath catheter proved easy to manipulate and performed as well as the IVUS catheter. During the coronary measurements no significant changes in arterial blood pressure (systolic arterial pressure 138±30 mmHg, diastolic arterial pressure 71±13 mmHg, mean arterial pressure 97±20 mmHg) or heart rate (65±13 bpm) were observed. During the procedure and up to one month clinical follow-up no patient experienced an adverse event.
Lumen measurements
Measurements on-line in 22 coronary stents with 46 subsegments (7 mm each) showed that average stent diameter was 2.57±0.31 mm by QCA, 2.81±0.31 mm by IVUS, and 2.91±0.29 mm by MC (P < 0.001 QCA versus MC, P = 0.13 IVUS versus MC). Results of on-line area measurements showed a small but significant difference between IVUS and MC (6.16±1.36 mm2 versus 6.72±1.31 mm2, respectively, P < 0.05). Individual data averaged per stent are summarized in Tables 2 and 3. Mean data for all three methods are presented in Table 4.
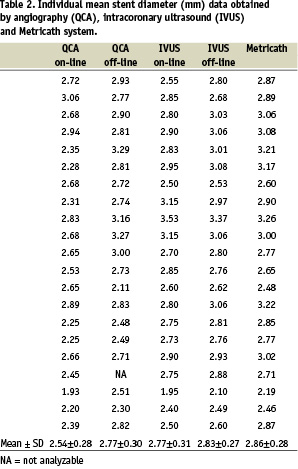
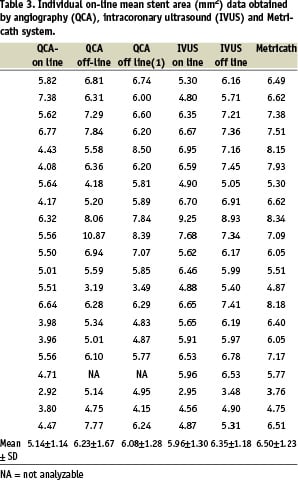
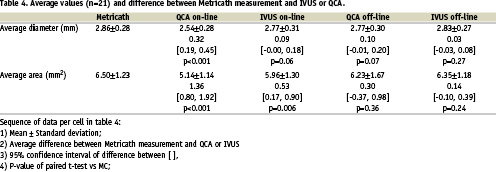
Regression analysis showed that MC correlated best with off-line IVUS (Fig. 2) both for area measurements (y=1.02x, R=0.90, P<0.001), and for diameter measurements (y=1.01x, R=0.91, P<0.001). The correlation was less with on-line measurements for both area (MC-IVUS: y=1.08x, R=0.80, P<0.001; MC-QCA: y=1.23x, R=0.46, P=0.037) and diameter (MC-IVUS: y=1.03x, R=0.76, P<0.001; MC-QCA: y=1.12x, R=0.48, P=0.028).
Figure 2. Linear regression analysis. Upper panels show regression analysis of area measurements between Metricath and IVUS (left), and Metricath and QCA (right). Lower panels show regression analysis of diameter measurements between Metricath and IVUS (left), and Metricath and QCA (right). To validate sufficient agreement between the methods, the line y=x was forced, and corresponding regression coefficients determined.
Bland-Altman analysis showed a mean difference in diameter between on-line MC and off-line IVUS of 0.04 ± 0.14 mm and between MC and off-line QCA of 0.10 ± 0.28 mm. Differences with on-line measurements were, for both techniques, more than double (Fig. 3).
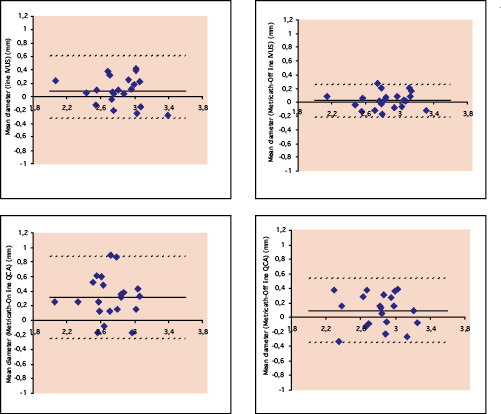
Figure 3. Bland-Altman plots. Upper panels show the comparison of diameter measurements between Metricath and on-line IVUS (left) and Metricath and off-line IVUS (right) Lower panels show the relationship for diameter measurements between Metricath and on-line QCA (left), and Metricath and off-line QCA (right). The continuous line indicates the average difference between the methods, the dotted lines the 95% confidence intervals.
Reproducibility of MC measurement was evaluated using pairwise sequential observations in both distal and proximal segments. Regression analysis (Fig. 4) showed a very strong linear relationship between the repeated pairs of Metricath System diameter measurements, i.e. y=1.03x - 0.05 (R=0.99, p<0.0001). The 95% confidence interval for the intercept was -0.17 to 0.03 and for the slope, 1.00 to 1.07.
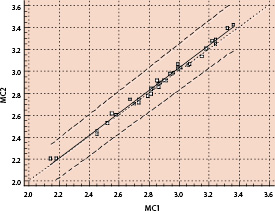
Figure 4. Linear regression of Metricath pair-wise repeat measurements.
Discussion
The accurate measurement of coronary lumen dimensions is of great importance in both diagnostic and interventional contexts. Furthermore, in an interventional setting, the final lumen has been shown to be of predictive value for the acute and long-term outcome10-17. Quantitative coronary angiography is the most widely used method to assess lumen dimensions due to availability and ease of use. However, it has well known limitations: accurate measurements can be hampered by sidebranch overlap, geometric distortion and foreshortening18. Furthermore, it underestimates lumen dimensions compared to IVUS, the gold standard.
The major finding of our study was that evaluation of stented lumen dimensions with Metricath, a novel non-imaging technique previously validated in experimental models, provided results that were comparable to those obtained with IVUS measurements performed in a Core Laboratory setting.
Study design and main findings
For the initial clinical evaluation of Metricath, we aimed at a coronary environment allowing for fixed lumen dimensions. It was assumed that in the stented coronary artery lumen size and geometry can be considered stable over the entire length of the stent over the time course of the protocol. Furthermore, the target site is easily visible due to the radiopaque stent struts. This guarantees that all measurements were performed at the same site within the coronary artery. Measurement of stent dimensions with Metricath was feasible, safe and reliable. The Metricath system showed good catheter flexibility and trackability, analysis proved possible in all arteries, and especially no macroscopic vessel injury occurred. Overall, there was good agreement and high correlation in lumen area and diameter dimensions within the stents between Metricath and the gold standard off-line IVUS. The two methods currently in clinical use (on-line QCA and IVUS) showed less agreement and a tendency towards underestimation of lumen dimensions by angiography with a mean area difference between the techniques of 0.83 mm2. The difference between QCA and IVUS as measured in the present study was very similar to those reported in diseased human coronary arteries19-22. Comparison of the new Metricath system measurements with both these techniques showed small and similar differences. There seems to be a tendency towards higher values obtained by Metricath as compared to QCA and IVUS. We do not know the reason for this, but it may be related to the fact that Metricath is the only method of the three studied with direct instrument-vessel wall interaction. Furthermore, compared to QCA, Metricath provides a direct measure of cross sectional area and does not assume vessel circularity. In addition, we cannot exclude differences in sampling. Sampling error in combination with inhomogeneous stent expansion, with smaller lumen at a distinct portion of the stent could result in differences between the methods. However, the impact of this possibility should be small, considering the requirement of <15% residual diameter stenosis for stent implantation to be regarded successful.
The observed difference between Metricath and IVUS and QCA are relatively small and within the range of variability for IVUS measurements7. The inter- and intra-observer difference for IVUS area measurements within stents is approximately 0.20 mm2, and the long-term reproducibility is in the range of 0.23 mm2.23 Our observation of a 0.14mm2 difference between IVUS and Metricath is thus well within the range of equivalent methods.
Potential clinical applications
Final lumen dimensions after percutaneous intervention have predictive value for the acute11-13 and long-term14,15 outcome. More widespread application of IVUS is impeded by the high costs of the ultrasound hardware and the imaging catheter and the need for trained staff for image interpretation. Furthermore, the additional value of IVUS over and above other measurement systems with respect to outcome is still unsettled16,17. The advent of drug-eluting stents (DES) has resulted in a remarkable decrease in restenosis in selected lesion/patient subsets. Observational studies using IVUS suggest that stent underdeployment plays a major role in in-stent restenosis and thrombosis in DES and have demonstrated that when such restenosis occur, event free survival is poor24,25.
Metricath potentially offers an easy to use alternative to IVUS; in addition, a combined Metricath and balloon angioplasty catheter might be of considerable practical value and could be produced at considerably lower cost than current IVUS technology.
Limitations
This study is limited by the relatively small number of observations and the narrow range of arterial dimensions evaluated. However, the target vessel dimensions represent the majority of clinically relevant dimensions in patients with coronary artery disease.
Image acquisition and pressure-volume curve analysis was not adjusted for the cardiac cycle. Arterial dimensions are known to change during the heart cycle in coronary arteries without stents26-28. In stented arteries, however, the scaffolding properties of the stent will diminish the cyclic variation of lumen dimensions.
Metricath balloons used in the present study were 7 mm in length. Therefore, it was not possible to determine areas over a shorter segment, for instance to identify minimal areas. Optimal balloon length will evolve over time with input from more clinical studies.
We analyzed stented coronary segments. Therefore, our data need to be substantiated for extrapolation in complex geometry, especially in non-stented atherosclerotic arteries.
Conclusion
The differences in stent measurement between Metricath and the gold standard off-line IVUS were small. Considering the accuracy and reproducibility and ease and rapidity of obtaining Metricath results, this technique may form an alternative to evaluate vessel area and stent expansion.
Appendix A
Deriving the Metricath equation
At a specific balloon fluid pressure, the volume of fluid infused into a balloon catheter - whose non-compliant balloon is unrestrained - is given as:


