Abstract
Aims: Paclitaxel-eluting stents (PES) have been proved safe and effective in selected patients undergoing percutaneous coronary interventions (PCI). However, there is uncertainty on the performance of PES in real-world patients at higher risk for major adverse cardiovascular events (MACE) or restenosis. We conducted a multicentre registry enrolling very high-risk subjects treated with PES.
Methods and results: We enrolled 1,065 consecutive patients undergoing PES implantation, provided that the target lesion treated with the PES was an unprotected left main (N=113), a true bifurcation (N=219), a chronic total occlusion (CTO, N=183), a long lesion (>28 mm, N=283), in a small vessel (<2.75 mm, N=417), or the patient had medically-treated diabetes mellitus (N=315). Clinical events were adjudicated at 1 and 7 months, and 4 to 8-month angiographic follow-up was recommended for core-lab quantitative coronary angiography. The primary end-point was the 7-month occurrence of MACE, i.e., the composite of cardiac death, non-fatal myocardial infarction (MI), coronary artery bypass grafting (CABG) and percutaneous target vessel revascularisation (TVR). A total of 2,116 lesions were treated with 2.0±1.2 Taxus per patient and 46.4±30 total Taxus length per patient. One total Taxus length per patient. One-month MACE occurred in 4.3% of patients, with 0.4% cardiac death, 3.3% myocardial infarction (MI), 0.1% coronary artery bypass grafting (CABG), and 0.8% target vessel revascularisation (TVR) PCI. Seven-month events were as follows: MACE 20.4%, cardiac death 1.2%, MI 4.2%, CABG 1.2%, TVR-PCI 15.4% and target lesion revascularisation (TLR)-PCI 10.9%. Binary restenosis occurred in 20.7% out of the 1,071 lesions undergoing follow-up angiography. Finally. stent thrombosis (ST) was reported with a 0.8% 12-month cumulative rate (0.3% acute, 0.3% subacute, and 0.2% <6 months, but no thrombosis >6 months).
Conclusions: This registry, enrolling 1,065 high-risk patients treated with PES, confirms the satisfactory performance of this device, especially given the overall profile of enrolled subjects and the limited number of stent thromboses.
Introduction
There is now evidence on the effectiveness and mid-term safety of drug-eluting stents in comparison to bare-metal stents (BMS) in selected patients and lesions.1 While new devices have been entering the market recently,2 most data concern polymer-based sirolimus-eluting stents (Cypher, Cordis, Miami, FL, USA) and polymer-based paclitaxel-eluting stents (PES, Taxus, Boston Scientific, Natick, MA, USA), and, indeed, since the publication of the first human feasibility study in humans in January of 2003, PES have entered the mainstream of interventional cardiology.3-6
While randomised trials enrolling subsets of higher-risk patients and lesions treated with PES have reported encouraging results, and awaiting for the ongoing trials of coronary artery bypass grafting (CABG) vs PES in patients with triple vessel or unprotected left main disease, the interventional cardiology community still faces the challenge of either avoiding the potentially beneficial implantation of a PES in a patient not strictly fulfilling the stringent selection criteria of the available randomised trials, or deciding for implanting a PES, despite the lack of a sound evidence base. In this context, we must rely on multicentre registries and non-experimental studies,7-8 notwithstanding their inherent limitations.9 Indeed, to date, only registries can provide the opportunity to test the risk-benefit ratio of PES in very high-risk or complex lesions unlikely to be the object of randomised comparison with BMS, such as unprotected left main, bifurcation lesions, or saphenous vein grafts.10-13
We thus conducted a multicentre registry enrolling high-risk patients and lesions treated with PES, with the goal of thoroughly appraising its mid-term risk-benefit profile both clinically and angiographically.14
Methods
Patients
Consecutive patients undergoing PES implantation, using the Taxus Express2 device were prospectively enrolled provided that the target lesion treated with the PES was an unprotected left main, a true bifurcation, a chronic total occlusion, a long lesion (>28 mm), in a small vessel (<2.75 mm), or the patient was diabetic. Exclusion criteria were: ongoing or recent (< 24 hours) ST-elevation acute myocardial infarction, impossibility to assume or continue to combined antiplatelet therapy (aspirin plus ticlopidine or clopidogrel) for at least eight months following PES implantation, or allergy to paclitaxel. High-risk patients treated at participating centres were not treated universally with PES, but occasionally, and at the physician’s discretion, with other devices. Nonetheless, most high-risk procedures during the study period were performed with this device. All enrolled patients provided written informed consent. Given the observational design, Ethics Committee approval was waived for data collection only.
Procedure
Coronary angioplasty and PES implantation were performed according to standard practice. At the start of the procedure, unfractionated heparin was recommended at a dosage of 70-100 IU/kg to achieve an activated clotting time > 250 seconds. Patients were started on aspirin and thienopyridines > 3 days before the procedure and to continue for > 8 months, while a loading dose of clopidogrel was used in those not previously taking thienopyridines.15
Clinical follow-up was scheduled for all patients by means of direct visit or phone call at discharge, and at one and seven months. Angiographic follow-up was recommended between four and eight months or earlier if a non-invasive evaluation or the clinical presentation suggested the presence of recurrent myocardial ischaemia.
Quantitative angiographic analysis
Coronary angiograms were analysed in a core laboratory (Mediolanum Cardio Research) according to standard methods (QCA CMS 5.2, Medis, Leiden, The Netherlands). Reference vessel diameter, minimum lumen diameter, diameter stenosis, lesion length and late loss were computed at the in-segment, proximal and distal (5 mm) edges, and in-stent. Binary angiographic restenosis was adjudicated in case of > 50% diameter stenosis.
Outcomes
The primary endpoint was the 7-month rate of major adverse cardiovascular events, defined as the composite of cardiac death, non-fatal myocardial infarction, coronary artery bypass grafting (CABG) and percutaneous target vessel revascularisation. As secondary endpoints, we analysed the individual 7-month occurrence of cardiac death, myocardial infarction, target vessel revascularisation, target lesion revascularisation, and stent thrombosis. Safety endpoints were the rates of major and minor bleeding (defined according to the Thrombolysis In Myocardial Infarction [TIMI] scheme), stroke, and bone marrow toxicity due to thienopyridines. Myocardial infarction was defined as Q-wave or non-Q-wave (defined as elevation of total CK 2 times above the upper limit of normal with a positive MB fraction in the absence of pathological Q waves), with all patients having post-procedural blood draws until discharge. Target lesion revascularisation was defined as any percutaneous revascularisation performed on the treated segment, and target vessel revascularisation as any percutaneous reintervention performed on the treated vessel. Stent thrombosis was adjudicated according to the definite and probable Academic Research Consortium definitions and distinguished in acute (occurring in the catheterisation laboratory), subacute (< 1 month) and late (>1 month).8
Statistical analysis
Continuous variables were reported as mean±standard deviation or median (interquartile range) and compared with Student t test or Mann-Whitney U or Wilcoxon tests, when appropriate. Categorical variables were reported as n/N (%) and compared with uncorrected chi-square or Fisher exact tests, when appropriate. The Kaplan-Meier method was employed for survival analysis. Multivariable logistic regression analyses were performed by concomitantly entering all significant (p<0.05) univariate predictors of adverse events in the final model. Similarly, we took into account time to event and censoring with Cox proportional hazard analyses. Computations were performed with BMDP (Saugus, MA, USA) and SPSS 11.0 (Chicago, IL, USA), with significance was set at the 2-tailed 0.05 level.
Results
Baseline characteristics and in-hospital outcomes
Patient, lesion and procedural characteristics are available in Tables 1-3.
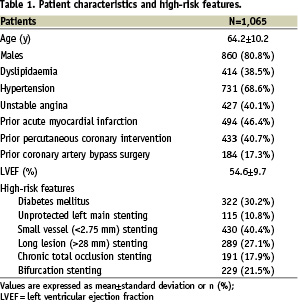
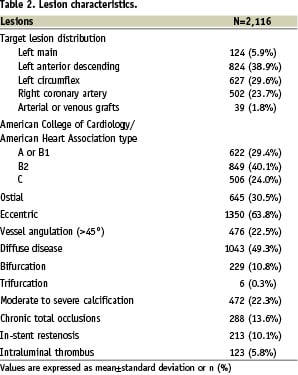
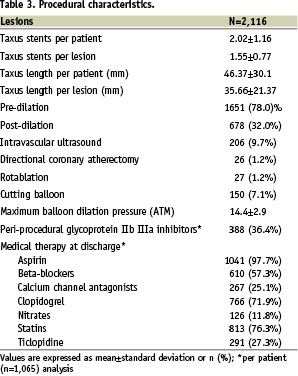
Specifically, enrolment according to the high-risk inclusion criteria is confirmed by occurrence of diabetes in 322 (30.2%), subjects, unprotected left main intervention in 115 (10.8%), true bifurcation stenting in 229 (21.5%), PES implantation in a chronic total occlusion in 191 (17.9%), in small vessels in 430 (40.4%), and in long lesions in 289 (27.1%). In-hospital major adverse cardiovascular events occurred in 40 (3.8%) patients, with two (0.2%) cardiac deaths, 32 (3.0%) myocardial infarctions, five (0.5%) target vessel revascularisations, and no CABG. In-hospital bleeding occurred in 15 patients (1.4%), with four (0.4%) major and 11 (1.1%) minor bleeds.
Mid- and long-term clinical outcomes
Clinical follow-up was available at seven and 12 months in 969 (91.0%) and 883 (82.9%) patients, respectively, and is reported, together with 1-month follow-up, in Table 4.
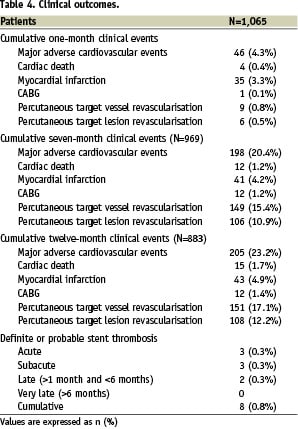
Specifically, median follow-up duration was 12.2 months (1st-3rd quartiles 11.9-13.0). Despite the complex patient and lesion features, major adverse cardiovascular events occurred at seven months in 198 (20.4%) patients, and most of them (149 [15.4%]) were percutaneous repeat revascularisations, respectively 37 (3.8%) on the target vessel but far from the target lesion, and 106 (10.9%) on the target lesion. Accordingly, CABG was needed in only 12 subjects (1.2%). Figure 1 shows the overall major adverse cardiovascular event-free survival according to the Kaplan-Meier method.
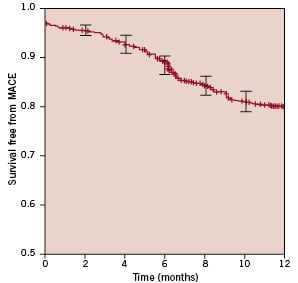
Figure 1. Survival free from major adverse cardiovascular events (MACE, i.e., cardiac death, non-fatal myocardial infarction, or target vessel revascularisation, either surgical or percutaneous), with 95% confidence intervals.
In addition, the favourable performance of PES in this complex setting was confirmed by the remarkably low rate of definite or probable stent thrombosis (cumulative 0.8%, acute 0.3%, subacute 0.3%, and late (>1 month) 0.2%. Notably, no case of stent thrombosis between six and 12 months was reported. Stent thrombosis defined as definite only was similarly uncommon (cumulative 0.7%, acute 0.3%, subacute 0.2%, and late 0.2%). Figure 2 shows survival free from thrombosis-related events (cardiac death, myocardial infarction, or stent thrombosis) according to the Kaplan-Meier method.
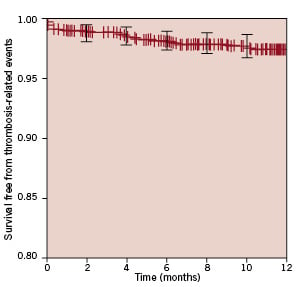
Figure 2. Survival free from thrombosis-related events (i.e., cardiac death, myocardial infarction, or probable stent thrombosis), with 95% confidence intervals.
Angiographic follow-up
Baseline and post-procedural core-lab angiographic analysis was completed on all cases. On the other hand, angiographic follow-up was available for 1,071 lesions (64.7% of those treated with PES). Details of such angiographic analysis are available in Table 5.
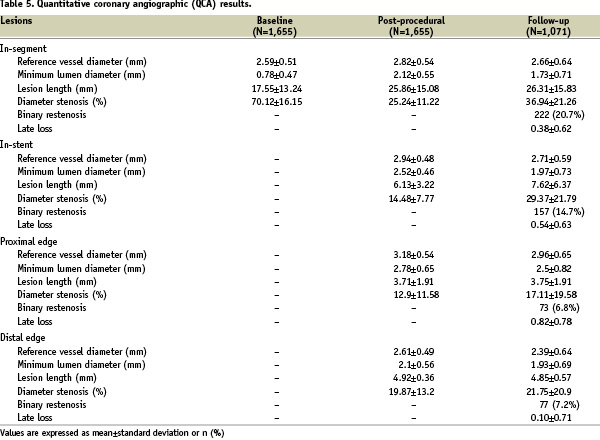
In particular, despite the high-risk characteristics of the lesions treated with PES in this study, late loss and binary restenosis rates appeared similar to those reported in trials employing PES in lower risk patients, with in-stent values of 0.54±0.63 mm and 14.7%, respectively.
Exploratory univariate and multivariable analyses
Univariate analysis identified the following predictors of MACE: number of diseased vessels (p<0.001), peripheral vascular disease (p=0.009), treatment of a bifurcation (p=0.034), a complex (p=0.027), or an ostial lesion (p=0.040), final TIMI flow <3 in any of the treated vessels (p=0.033), number of treated lesions (p<0.001), number of implanted PES (p=0.001), and per-patient PES length (p=0.002). Multivariable analysis, including these covariates simultaneously, disclosed that the only multivariable predictor of MACE was number of treated lesions (p=0.002). Results of logistic regression analysis were confirmed at Cox proportional hazard analysis (p<0.001 for number of treated lesions at multivariable analysis).
Among univariate predictors of death, we identified achievement of complete revascularisation (p=0.005), congestive heart failure (p<0.001) or acute coronary syndrome (p=0.002) at admission, diabetes (p=0.016), chronic renal failure (p<0.001), and left ventricular ejection fraction (p=0.026). Among these, only complete revascularisation (p=0.015), acute coronary syndrome at admission at admission (p=0.013), and chronic renal failure (p=0.001) remained as significant multivariable predictors of death.
Number of diseased vessels (p=0.009), peripheral vascular disease (p=0.010), target vessel (p=0.041), treatment of a chronic total occlusion (p=0.004), bifurcation (p=0.042), use of intravascular ultrasound (p=0.003), number of treated lesions (p=0.001), and final TIMI flow (p<0.001) were univariate predictors of myocardial infarction, while only treatment of a chronic total occlusion (p=0.036), number of treated lesions (p=0.001), and final TIMI flow (p=0.001) remained as multivariable independent predictors.
Univariate analysis identified the following predictors of TVR: prior infarction (p=0.036), number of diseased vessels (p=0.002), target vessel (p=0.003), number of treated lesions (p=0.001), per-lesion PES length (p=0.007), and per-patient PES length (p=0.011). Multivariable analysis including these covariates simultaneously disclosed that the only multivariable predictors of TVR were prior infarction (p=0.008) and target vessel (p=0.004), with a higher risk of TVR in lesions located in a left main or in a saphenous vein graft.
Finally, univariate analysis disclosed the following predictors of cumulative stent thrombosis: treatment of a bifurcation (p=0.035) or ostial lesion (p=0.050), reference vessel diameter (p=0.027), and maximum balloon dilation pressure (p=0.002). Multivariable analysis including these covariates simultaneously showed however that only ostial location (p=0.018) and maximum balloon dilation pressure (p=0.003) independently predicted stent thrombosis, with this event being more frequent in ostial lesions or after dilation with lower pressures. Specifically, lesions with stent thrombosis had been dilated at a median of 10 atm (1st-3rd quartiles 8-13), while lesions without thrombosis had been dilated at a median of 14 (12-16).
Discussion
Findings of the present multicentre registry, appraising the safety and effectiveness of PES in high-risk patients and lesions, are three-fold: a) in light of the complex clinical and anatomical setting, the performance of the device was satisfactory, with the vast majority of events due to repeat percutaneous revascularisation procedures and only 12 cases CABG at 12-month follow-up; b) optimal antithrombotic regimens, including pre-procedural thienopyridine pretreatment or loading, peri-procedural glycoprotein IIb/IIIa usage at physician’s discretion, and long-term (> 8 months) double antiplatelet treatment, yielded low rates of stent thrombosis and other thrombosis-related events, a remarkable achievement given the very complex patient and lesion setting; c) the powerful anti-proliferative effect of PES was confirmed even in these series of complex procedures, as shown by rates of binary restenosis and values of late loss obviously lower than those of historical bare-metal stent controls. Other important findings stemming from the TRUE registry have already been presented, including data on bifurcation lesions and thienopyridine treatment.15-16
Current clinical research context
Since their introduction, drug-eluting stents in general and PES in particular have been widely adopted by interventional cardiologists worldwide.1,3-4 However, as with most medical interventions, evidence of benefit for PES was at first limited to highly selected patients with relatively benign and not complex lesions.3-4 While such proof of effect provided the basis for the diffusion among interventionists, together with the results of the first pivotal trial, the TAXUS IV,5 uncertainty has persisted on the risk-benefit balance of this device in higher risk patients and lesions.
More recent randomised trials enrolling complex patients and lesions have been reported in support of the use of PES, including the TAXUS V and the TAXUS VI trials.17,18 specifically, the TAXUS V trial reported on 1,156 patients undergoing PCI in small, very large, or long lesions with either PES (n=577) or bare-metal stents (n=579) for a mean stent length per patient of 28 mm.17 At nine months, compared with bare-metal stents, PES reduced target lesion revascularisation from 15.7% to 8.6% and target vessel revascularisation from 17.3% to 12.1%. Similar benefits were found concerning binary angiographic restenosis, which was reduced from 33.9% to 18.9% in the entire study cohort, as well as among patients receiving 2.25-mm stents (49.4% vs 31.2%), 4.0-mm stents (14.4% vs 3.5%), and multiple stents (57.8% vs 27.2%). The TAXUS VI similarly enrolled 448 patients with a target coronary lesion length ranging from 18 to 40 mm, treating them with PES (n=219) or bare-metal stents (n=227) for a mean stent length per patient of 33 mm, showing at nine months a 9.1% rate of target vessel revascularisation in the PES group and 19.4% in the BMS group.19 Despite the novel data on higher-risk patients provided by these most recent TAXUS studies, even these trials were limited by strict selection criteria and obvious issues of external validity that is typical of randomised studies.19 Notably, chronic total occlusions, bifurcations, and left main interventions were explicitly excluded from these study, while they formed a major part of included cases in the true study.
Beside our current work, other carefully designed prospective observational studies have provided a thorough, and as far as possible, unbiased appraisal of the risk-benefit balance of PES in TRUE high-risk patients. In this context, precise and complete data are evolving, especially given the fact that PES was only introduced into clinical practice a few years ago and thus long-term follow-up is incomplete. Nonetheless, Ong et al reported on unselected patients treated with PES (N=576) or sirolimus-eluting stents (N=508) in the Thoraxcenter, Rotterdam.7 At 1-year follow-up, major adverse cardiovascular events occurred in 13.9% and 10.5% respectively, with an adjusted hazard ratio of 1.20 (0.85 to 1.70), while clinically driven target vessel revascularisation was 5.4% versus 3.7%, respectively (hazard ratio 1.38 (0.79 to 2.43). Despite the absence of explicit selection criteria, in this study only 4% of subjects were treated for left main disease and 16% for bifurcation lesions, with a total stent length per patient of 42.9 mm, thus making their patient population at a lower risk than that of the TRUE study. Similar results have been reported in larger post-marketing surveillance studies, such as the ARRIVE, STENT, and WISDOM registries.
Limitations of the present study
Drawbacks of non-randomised trials are well known,2 and are also pertinent to the TRUE Registry. Of utmost relevance to the current debate on the safety of drug-eluting stents20-24 is the length of follow-up, which is mainly limited to the 7-month frame in this work, and thus likely underestimates event rates, specifically stent thrombosis. Thus, at this time, no definitive conclusions on the risk-benefit balance of PES after one year in high-risk patients similar to those enrolled in the TRUE study can be drawn. In addition, angiographic follow-up was recommended on a clinical basis, given the high-risk patient characteristics, but even patients not willing to undergo angiographic follow-up could be enrolled. This leads to a relatively low angiographic follow-up rate, at least when compared to randomised trials with systematic angiographic control.3-4 In addition, PESs were implanted at the physician’s discretion, thus patient inclusion is representative of current treatment practices at participating centres. Finally, the lack of a clinical event committee for all events should be viewed with caution, even if angiographic analyses and adjudication of target vessel events were performed by an independent core laboratory.
Conclusions
The TRUE multicentre prospective study, enrolling more than 1,000 patients with complex clinical or lesion features undergoing PES implantation, supports the favourable mid-term performance of this drug-eluting stent, especially given the profile of enrolled subjects and the limited number of stent thromboses.
Funding source
Supported by Boston Scientific Italia, Genova, Italy.

