Abstract
Aims: Our prospective study sought to investigate whether inadequate platelet responses to clopidogrel can be corrected by increasing the daily maintenance dose from 75 mg to 150 mg.
Methods and results: In 117 patients with elective PCI after loading with 600 mg clopidogrel, we determined residual platelet aggregation in response to 5 µmol/l ADP (RPA) by optical aggregometry after the first 75 mg maintenance dose (baseline), and at days 14 and 28. In patients with RPA >14% at baseline, we increased the daily dose to 150 mg. Fifty-seven additional patients without dose adjustment served as historic control. In 39 patients with baseline RPA >14%, the increase in maintenance dose to 150 mg reduced median RPA significantly (P<0.001) from 24% [interquartile range: 18-32%] at baseline to 14% [8-20%] at 14 days without any further significant change. In patients with RPA < 14%, who continued on 75 mg clopidogrel, RPA increased during the first 14 days by 4.5% (0-14%; P<0.001). At 14 days, the study group with selective dose adjustment had a significantly lower RPA than the control group without dose adjustment (10.0% [4-20%] versus 17.0% [9-32%], P<0.001).
Conclusions: In patients with a low initial response to clopidogrel, platelet inhibition can be improved by increasing the maintenance dose to 150 mg.
Introduction
Platelet inhibition by clopidogrel shows a broad variability, both after loading and during maintenance therapy1-4. Several independent studies suggested that the risk of adverse cardiac events after percutaneous coronary intervention (PCI) critically depends on the level of platelet inhibition achieved in response to clopidogrel5-9. Specifically, the Impact of Extent of Clopidogrel-Induced Platelet Inhibition During Elective Stent Implantation on Clinical Event Rate (EXCELSIOR) study showed a more than 3-fold increase in the risk of death and myocardial infarction during the first year after stent placement, if residual platelet aggregation in response to 5 µmol/l adenosine diphosphate (ADP) was above 14% after the first 75-mg maintenance dose of clopidogrel following loading with 600 mg one day before10.
Platelet aggregation after stimulation with ADP is blocked incompletely after administration of loading doses up to 600 mg clopidogrel11 or during maintenance dosing regimens12. This observation has been attributed to an incomplete inhibition of the ADP-binding sites at the P2Y12 receptor13. Previous studies showed that clopidogrel as well as ticlopidine produced only partial inhibition of [3H]-2-methylthio-ADP binding to platelets14,15. High on-treatment platelet reactivity after clopidogrel may be related to a total or partial inability to convert clopidogrel into the active metabolite16 or an increased baseline platelet reactivity10,17. The ISAR-CHOICE 2 study18 as well as the OPTIMUS study19 randomised patients to daily doses of either 75 or 150 mg clopidogrel and demonstrated an increase in antiplatelet effect by the higher maintenance dose. In contrast to the aforementioned studies we aimed to investigate, if and to what extent an inadequate antiplatelet response to clopidogrel can be corrected by increased dosing specifically in patients with inadequate initial antiplatelet response during maintenance therapy.
To address this question, we conducted the EXCELSIOR-Adjusted Clopidogrel Treatment (ACT) study. This prospective observational cohort study tested whether residual platelet aggregation in patients with inadequate response to clopidogrel after the first 75-mg maintenance dose can be substantially improved by an increase in maintenance dose to 150 mg.
Furthermore, we compared residual platelet aggregation in the EXCELSIOR-ACT cohort with selective dose adjustment in addition to platelet aggregation in a historic subgroup from the EXCELSIOR study without any dose adjustment. This historic control group consisted of 57 patients of the EXCELSIOR trial with platelet function testing at 14 and 28 days. The entry criteria of EXCELSIOR were identical to those of EXCELSIOR-ACT, but all patients continued on the 75-mg maintenance dose, irrespective of the results of platelet function testing performed two to four hours after administration of the first maintenance dose of 75 mg clopidogrel at day one after PCI.
Methods
Study population
Patients undergoing elective coronary stent placement after pre-treatment with 600 mg of clopidogrel and aspirin (> 100 mg per day for at least five days) were eligible for the study. Major exclusion criteria were acute myocardial infarction according to the American Heart Association/American College of Cardiology (AHA/ACC) criteria, chronic oral anticoagulation, thienopyridine treatment within the last 2 weeks before admission, use of a glycoprotein IIb/IIIa inhibitor within the last seven days or during study period, contraindications to aspirin, clopidogrel or heparin, cancer, haemodialysis and haemodynamic instability. Concomitant treatment with strong inhibitors (such as macrolide antibiotics, azole antifungals) or inducers (such as rifampicin) of cytochrome P450 3A4 was not permitted. All patients gave written informed consent. The study complies with the Declaration of Helsinki and was approved by the ethics committee of the medical faculty of the University of Freiburg, Germany.
Before the intervention, all patients were on aspirin and received a loading dose of clopidogrel 600 mg. Catheterisation and stent placement was performed according to current standard guidelines. All patients received an intra-arterial dose of 100-140 U/kg heparin. The choice of stent type was left to the operator’s discretion. After PCI, the vast majority of patients were treated with aspirin 100 mg per day for at least the duration of the study. Only one patient received 300 mg aspirin daily. We administered the first 75-mg maintenance dose of clopidogrel in the morning of the day following PCI, i.e., one day after loading. The blood sample for platelet function testing was obtained between two to four hours after intake of the first maintenance dose. Patients with a residual platelet aggregation >14% in response to 5 µmol/l ADP received a further bolus dose of clopidogrel 300 mg followed by an increased daily dose of 150 mg (75 mg bid). No further dose adjustments were performed. If residual platelet aggregation at baseline was < 14%, patients continued on the 75-mg maintenance dose. The threshold of 14% for the 5 µmol/l ADP-induced RPA at 24 hours after PCI was identified by EXCELSIOR as predictive for subsequent adverse cardiac events7. We scheduled all patients for follow-up visits at 14 and 28 days after PCI for re-evaluation of platelet function. Concomitant medication was not altered during the study. To check compliance to antiplatelet treatment, we interviewed the patients and performed pill counting.
Platelet function assays
Blood was drawn for platelet function assays using tubes containing 3.8% sodium-citrate (S-Monovetten, Sarstedt AG, Nuembrecht, Germany). Platelet aggregation was assessed by turbidimetric aggregometry using a four-channel Bio/Data PAP4 aggregometer (Moelab, Langenfeld, Germany), as described previously7. We prepared platelet-rich plasma by centrifugation of blood samples (750 g/2 minutes) and adjusted to 275-325x109 thrombocytes/l by dilution with platelet poor plasma from the same patient. Results were expressed as percentage of maximal light transmission determined five minutes after stimulation with ADP 5 µmol/l using platelet poor plasma as reference (=100% aggregation). We elaborated on late or residual platelet aggregation (RPA) because previous experiments found a lower rate of variability at stabilisation of aggregation, measured by disaggregation and late aggregation, which is mainly driven by the activity of P2Y12 receptor20. Peak aggregation used in some of the previous studies4,21,22 is influenced by the activity of both the P2Y1 and P2Y12 purinergic receptors, whereas clopidogrel blocks only the latter. The coefficient of variation of our optical aggregometry assay is 6.1%3. Laboratory personnel were blinded to treatment assignments.
Statistics
The primary endpoint of EXCELSIOR-ACT was the decrease in residual platelet aggregation after adjustment of the maintenance dose of clopidogrel to 150 mg. We designed the study to have a power of 80% to detect a 20% decrease in residual platelet aggregation with a level of significance of P < 0.05. Based on the EXCELSIOR-substudy we assumed an initial residual ADP-induced platelet aggregation of 35±15% for the group with platelet aggregation >14%. With these assumptions we obtained a sample size of 39 patients with dose adjustment (nQuery Advisor, Statistical Solutions, Cork, Ireland; version 5.0).
In general, we report discrete variables as counts (percentages) and continuous variables as mean ± standard deviation. Unless stated otherwise, we tested differences between groups with the χ2-test or Fisher’s exact test (for estimated cell sizes < 5) for discrete variables and with one-way ANOVA for continuous variables. Because of the non-normal distribution of residual platelet aggregation (Kolmogorov-Smirnov test) we report platelet data as median (interquartile range). We used the Wilcoxon signed rank test to assess the changes in platelet aggregation between two time points and the Mann-Whitney-U test to compare the EXCELSIOR-ACT and the EXCELSIOR-substudy cohorts with respect to platelet function. To corroborate our non-parametric tests, we analysed paired platelet function data by the t-test for paired samples. We also compared the change in ADP-induced residual platelet aggregation between the study group and the control group with and without adjustment for differences in baseline characteristics at P < 0.1 (Table 1) by calculating univariable and multivariable general linear models. For all statistical analyses, we used the SPSS software package (SPSS, Chicago, IL, USA; version 14). In the two-tailed test, a P-value < 0.05 was regarded as significant.
Results
The study flow-chart is provided in Figure 1.
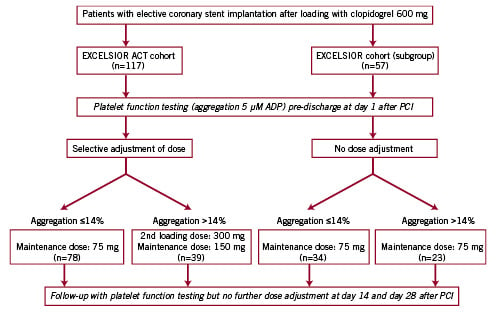
Figure 1. Study flow-chart
The EXCELSIOR-ACT cohort comprised 117 consecutive patients. Baseline characteristics of these patients are shown in Table 1. As shown in Table 1 and in Figure 2, 39 patients (33%) exhibited a residual ADP-induced platelet aggregation >14% after the first 75-mg maintenance dose of clopidogrel.
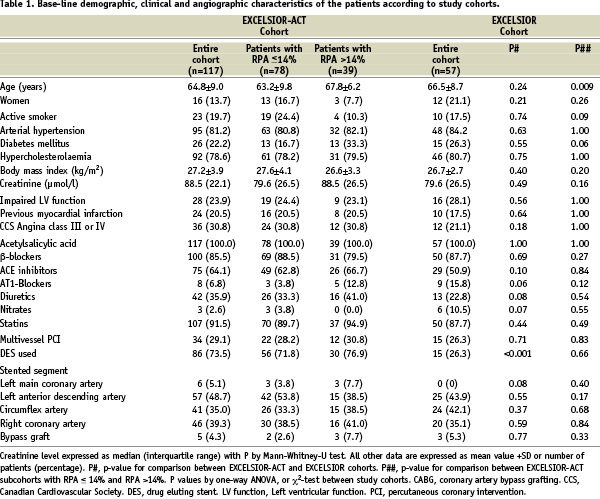
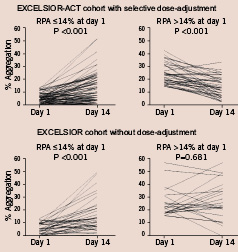
Figure 2. Graphs showing individual RPA from day one to day 14 in patients from the EXCELSIOR-ACT and the EXCELSIOR cohort. Patients were stratified according to RPA at day one. Patients with RPA >14% at day one (upper right panel) were treated thereafter with clopidogrel 150 mg daily, while all remaining patients received clopidogrel 75 mg per day throughout. P-value by Wilcoxon signed rank test.
Increasing the maintenance dose to 150 mg in these patients significantly reduced ADP-induced RPA by 9.0% (5.0 to 15.0%; median and interquartile range) from a median of 24.0% (interquartile range: 18.0-32.0%) after the first maintenance dose to a median of 14.0% (interquartile range: 8.0-20.0%) at 14 days (P < 0.001; Figures 2 and 3). At day 28, platelet aggregation was not significantly different from that at day 14 (Figure 3).
Patients with an initial ADP-induced RPA < 14%, who continued on the 75-mg maintenance dose, showed a slight, but statistically significant increase in RPA during the first 14 days without any further significant changes from day 14 to day 28 (Figures 2 and 3). At day 14, ADP-induced RPA in patients with a blunted response to clopidogrel, but maintenance-dose adjustment, still had a significantly higher level of RPA than those with an adequate response to clopidogrel and no maintenance dose adjustment (P=0.040, Figure 3).
The baseline characteristics of the 57 patients of the historic control group from the EXCELSIOR-substudy are shown in Table 1. Compared with the EXCELSIOR-ACT cohort, the EXCELSIOR-substudy cohort received fewer drug eluting stents. Regarding concomitant medication, trends for a more frequent use of nitrates and angiotensin 1 receptor blockers and a lower prescription rate of diuretics and ACE-inhibitors were observed in the EXCELSIOR-substudy cohort. In the entire EXCELSIOR-substudy cohort there was a statistically significant (P < 0.001) increase in ADP-induced RPA from a median of 10.0% (interquartile range: 4.0-20.0%) after the first maintenance dose to a median of 17.0% (interquartile range: 9.0-32.0%) at 14 days (Table 2).
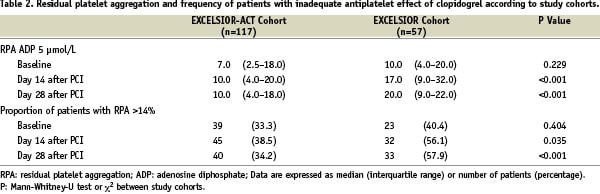
Comparing the EXCELSIOR-substudy cohort with the recent EXCELSIOR-ACT cohort, we found a significant effect of the increase in maintenance dose in patients with an ADP-induced RPA >14% at study entry with regard to RPA at day 14: Among patients with a blunted response to clopidogrel, the change in ADP-induced RPA differed significantly between the two patients subsets with (EXCELSIOR-ACT) and without increase in maintenance dose (EXCELSIOR), by –12.1% (95%-confidence interval: –18.1 to –6.1%, P < 0.001) without adjustment and by –11.0% (95%-confidence interval: –18.8 to –3.1%, P=0.007) with adjustment for the difference in the use of drug-eluting stents and use of angiotensin 1 receptor blockers, nitrates, diuretics and ACE-inhibitors. At 14 and 28 days, the cohort with selective increase of the maintenance dose to 150 mg (EXCELSIOR-ACT) showed a significantly lower overall level of ADP-induced RPA than the cohort with the fixed 75-mg maintenance dose. The proportion of patients of the EXCELSIOR-ACT cohort reaching the target of RPA< 14% was by 17.6% (day 14) and by 23.7% (day 28) higher than that in patients of the control group without selective dose adjustment (Table 2).
Discussion
As the main message of our study we demonstrate that doubling of the conventional maintenance dose of clopidogrel is an option to improve platelet inhibition in patients with an initially poor response to clopidogrel. In patients with a residual ADP-induced platelet aggregation >14% after the first 75-mg maintenance dose preceded by a 600-mg loading dose the day before, the increase in maintenance dose to 150 mg substantially reduced the steady-state level of RPA compared with continuation of the 75-mg maintenance dose. After doubling the maintenance dose of clopidogrel, ADP-induced RPA at 14 days in patients with a poor initial response was significantly lower than at pre-discharge, whereas RPA at 14 days in patients with an adequate initial response and no increase in maintenance dose was slightly but significantly higher than at pre-discharge. Thereby, the level of RPA in patients with a poor response and increase in dose improved markedly but was still significantly worse than that of patients with a good response but no dose adjustment. Similar findings were obtained in a small cohort of diabetics using a point-of-care assay for the assessment of antiplatelet effect23. In the overall cohort, monitoring of antiplatelet effect with selective dose adjustment of clopidogrel induced a more consistent and lower RPA than the routine administration of the approved 75-mg maintenance dose. Thus, more than 60% of our cohort displays an adequate response to clopidogrel during maintenance dosing of clopidogrel. The proportion of patients with optimal RPA (< 14%) in EXCELSIOR-ACT is substantially higher than in the OPTIMUS study (40%) which investigated patients with type 2 diabetes mellitus19. Although different definitions of adequate response were used it seems more likely that the lower responder rate in OPTIMUS can be attributed to the known enhanced platelet reactivity and the reduced in vitro responsiveness to antiplatelet agents in diabetics24,25.
Clinically, it is desirable to obtain an estimate of platelet function during maintenance treatment before discharge from hospital. The clopidogrel effect, however, has not yet reached a steady-state level at this time point. We found that the level of RPA determined between two and four hours after administration of the first maintenance dose of clopidogrel 75 mg at day one after PCI overestimates residual platelet aggregation during maintenance treatment by about 7.5%. This finding may be explained by a residual effect of the 600 mg loading dose administered before PCI, and presents additional circumstantial evidence that the antiplatelet effect of the 75-mg maintenance dose of clopidogrel is sub-maximal. Despite the overestimation of the chronic antiplatelet effect, the level of RPA measured between two to four hours after the patients received the first maintenance dose of clopidogrel at day one post-PCI is predictive of cardiac complications during the first year after PCI10. Therefore, pre-discharge assessment of platelet function may be clinically useful for tailoring subsequent maintenance therapy, as also suggested by two recently published studies26,27.
The threshold of 14% for the 5 µmol/l ADP-induced RPA assessed at that time after PCI was validated by EXCELSIOR as predictive for subsequent adverse cardiac events7,10. The level of RPA (14%) used in our study represented the 72nd percentile of the distribution of platelet aggregation of the EXCELSIOR cohort and the 67th percentile of EXCELSIOR-ACT. Hence, the level of RPA must be considered hazardous if it exceeds the upper tertile of the cohort. In the EXCELSIOR substudy, the upper tertile of RPA at 14 days was 24%. After the increase in maintenance dose in EXCELSIOR-ACT, 90% (35 of 39) of the patients with blunted response to clopidogrel at 24 hours reached a platelet aggregation below this threshold (Figure 3). Thus, in the majority of patients with dose adjustment platelet aggregation may be considered sufficiently suppressed. Nevertheless, doubling the maintenance dose of clopidogrel could not fully correct the low response to clopidogrel and a small number of patients did not respond to the increase in dose at all.
The first circumstantial piece of evidence which shed doubts on the adequacy of the approved maintenance dose of clopidogrel was the finding that re-administration of a loading dose of clopidogrel in patients already on maintenance treatment evokes further platelet inhibition28. Later on the same group presented data that doubling the maintenance dose to 150 mg early after PCI and loading with 600 mg clopidogrel improves the antiplatelet effect18. These results were confirmed most recently in a study using a similar design27. A previous randomised pilot study by Angiolillo and co-workers comprised 40 diabetic patients with a low response to clopidogrel in which antiplatelet effects were assessed during chronic treatment19. Consistent with our current findings, the increase in maintenance dose to 150 mg enhanced the antiplatelet effect. Apart from expanding the data base for the 150-mg maintenance dose, our study adds two important new aspects: (1) Pre-discharge assessment of platelet function after PCI is a sensitive tool to identify patients with a poor response to clopidogrel who benefit from an increase in maintenance dose. (2) Selective dose adjustment in one third of the patients suffices to suppress the level of RPA in the entire cohort substantially.
Clinical implications
The current study demonstrates that in many patients doubling the maintenance dose represents an option to correct an inadequate response to the 75-mg dose of clopidogrel. Moreover, our study suggests that monitoring the antiplatelet efficacy with selective dose adjustment of clopidogrel is a cost-effective means of achieving optimal suppression of platelet aggregation in the majority of patients. Although dedicated clinical studies are needed to confirm this concept, it is elusive that individualised dosing of clopidogrel with doubling of maintenance dose will improve the clinical outcome after PCI, particularly when drug-eluting stents have been used.
Increasing the maintenance dose of clopidogrel, however, does not always correct inadequate platelet inhibition. In those patients who do not reach a sufficiently low level of residual platelet aggregation despite increased maintenance doses, an alternative pharmacological approach to the inhibition of the P2Y12-receptor is warranted. Newer P2Y12-receptor antagonists that are currently investigated in phase III trials such as prasugrel and AZD-6140 appear promising29,30.
Limitations
The non-randomised design of the study represents a limitation for the comparison between the study group and the control group. Except for the use of drug-eluting stents which increased over time and the use of nitrates, there were, however, no major baseline differences between the EXCELSIOR-ACT cohort and the historical EXCELSIOR sub-study cohort. In EXCELSIOR we did not find any interaction between the placement of a drug-eluting stent and the effect of clopidogrel or treatment with nitrates. Moreover, to adjust for the placement of a drug-eluting stent and for differences in concomitant medication, we performed a multivariable analysis which confirmed the result of the non-adjusted comparison.
Our study did not address the question whether selective dose adjustment of clopidogrel improves the outcome after PCI with stent placement. EXCELSIOR demonstrated that an ADP-induced RPA above 14% is associated with a more than 3-fold increase in risk of death and myocardial infarction during 1-year follow-up10. Although EXCELSIOR-ACT showed that an inadequate response to clopidogrel can be corrected in a large proportion of patients by increasing the dose, the study did not have the power to assess the effect on clinical outcome. EXCELSIOR-ACT breaks the ground for larger studies that address the clinical efficiency of selective dose adjustment which will also have adequate statistical power to assess the bleeding risk associated with an increased maintenance dosing regimen of clopidogrel.
Some patients did not respond to the increase in the dose of clopidogrel to 150 mg. We did not test maintenance doses higher than 150 mg. Therefore, we cannot clarify whether non-response to 150 mg clopidogrel was caused by a principle resistance to clopidogrel or by a still insufficient dose.
Conclusions
The current study demonstrates that in a large proportion of patients treated with the approved maintenance dose of clopidogrel platelet activity is poorly suppressed. Early laboratory assessment of the antiplatelet effect of clopidogrel is a useful tool for identification of patients with an attenuated therapeutic response. Increasing the maintenance dose of clopidogrel to 150 mg only in patients with an inadequate initial antiplatelet response to clopidogrel may be a cost-effective approach to improve antiplatelet effects of clopidogrel during maintenance dosing. However, the clinical efficacy and safety of this approach remains to be investigated in the context of a large scale trial.
Acknowledgements
The study was supported by an unrestricted grant from Herz-Zentrum Bad Krozingen.

