Abstract
Aims: To assess the use of drug eluting stents (DES) and passive stents in patients undergoing percutaneous coronary interventions (PCI) in Europe.
Methods and results: We looked at the characteristics, procedural details and immediate outcome of 9,380 patients consisting of 2,471 STEMI patients (26.3%), 1,752 patients with NSTEMI-ACS (18.7%), and 5,157 elective patients (55.0%) in the European Heart Survey. The hybrid population of DES and passive stented patients (506 patients), and those treated without a stent, were excluded from the direct comparison between the two stented subgroup. These patients were, however, included in the total treated PCI population consisting of 10,982 patients.
Patients presenting with stable angina or NSTEMI-ACS were more often treated with DES than patients with STEMI, which was in accordance with the current European Society of Cardiology (ESC) guidelines. There was no excess of acute stent thrombosis in the DES treated patients in any clinical setting including STEMI. Periprocedural complications were less in STEMI patients treated with DES compared to passive stents (BMS or coated stents). More complicated lesions and off-label use was observed with DES than passive stents. The overall in-hospital mortality was low (1.8%) and was better in the DES (1.2%) than passive stented patients (2.1%). In-hospital MACCE for the total PCI population (N=10,982) reflected the clinical presentation as it increased from electives (1.4%), NSTEMI-ACS (6.4%) to STEMI (7.8%) patients.
Conclusions: There is marked variability in DES use across Europe. More detailed studies are needed to address the outcome of DES, especially in STEMI patients and the off-label use in order to contemplate a revision of the current ESC guidelines.
Introduction
The European Heart Survey (EHS) programme is aimed at providing up to date information on the current state of cardiovascular disease (CVD) in Europe1. In particular, to the adherence of published guidelines, whether or not patients can be enrolled in appropriate trials, and the differences in managing CVD amongst the member states. Since 1999 there have been a total of 12 completed surveys giving useful information on current clinical practices2. The most recent survey on percutaneous coronary intervention (2005-2006) became the PCI registry database. This followed the coronary revascularisation survey of 2001-2002 with bare metal stents (BMS).
Although several meta-analyses confirm the superiority of drug-eluting stents (DES)3-6 in reducing restenosis, there are now emerging concerns with regard to clinical events such as late stent thrombosis7-11. As such, the long-term behaviour of DES is yet to be determined. It is therefore vital to have accurate patient records of their implantation and subsequent follow-up, like those in registry databases to fully evaluate the effectiveness of these stents. We therefore investigated the EHS database to determine what population of patients are currently being treated with a DES or a passive stent (BMS or coated stents) in Europe and to see whether they comply to existing European Society of Cardiology (ESC) guidelines on DES implantation12. Our data was however limited to the DES and passive stent usage, and not to any long-term follow-up that will be subsequently reported by the EHS as data becomes available.
The ESC Guidelines on DES usage
The current guidelines for DES use are at a recommendation level of 1B for de novo lesions of more than 50% in the native coronary arteries of patients with stable or unstable angina or documented ischaemia. Lesion characteristics were chosen in accordance to the SIRIUS trial13,14 (involving the Cypher™ stent), with a reference diameter of 2.5-3.5 mm and a lesion length of 15-30 mm or, in the case of TAXUS-IV and TAXUS-V15 (regarding the Taxus™ stent), with a reference diameter of 2.5-3.75 mm and a lesion length of 10-28 mm. Patients with acute myocardial infarction (MI) or status post MI, bifurcations or ostial lesions, unprotected left main stem lesions, visible thrombus, severe tortuosity and/or calcification, were not included in the current ESC guidelines. The recommendation of DES is currently at level IIaC in patients with lesions in small vessels, chronic total occlusion, bifurcation and ostial lesions, coronary artery bypass stenosis, insulin-dependent diabetes mellitus, multivessel disease, unprotected left main stenosis and in-stent restenosis12,16.
Methods
The Euro Heart Survey on PCI involved 29 ESC member countries and 137 hospitals. A national coordinator was responsible for maintaining contact with the investigators in each of the participating centres, and for overseeing the implementation of the survey protocol. In each participating centre, a data collection officer was responsible for screening the patients admitted for a PCI. Data regarding the pre-hospital and in-hospital course, follow-up data were collected at 30 days. Patient identification was not recorded on the case report forms. The centres were instructed to keep a log of all included patients, in which their names, contact information, and study code were recorded, in order to enable follow-up. Electronic case report forms were used for data entry and transferred via the web to a central database located in the European Heart House, where they were edited for missing data, inconsistencies, and out-liers.
The patient population
The EHS data were collected in accordance with the Cardiology Audit and Registration Data Standards (CARDS). The programme on PCI in Europe covered the period May 2005 to December 2005 and included 14,575 patients from 29 ESC member countries and 137 hospitals. The data included both acute and elective patients and a subgroup of patients (25%) defined as post-STEMI, post-NSTEMI-ACS and post-unstable angina. These were patients in whom PCI followed various degrees of stabilisation after the initial event and thus differed from the elective patient population with stable angina. As they were neither non-acute nor elective patients we have not included them in our analyses, but have chosen to concentrate particularly on those with STEMI, NSTEMI-ACS or stable angina, hereafter called the treated patients. This subgroup had a total of 10,982 patients with 2,753 STEMI patients (25.1%); 2,036 patients with NSTEMI-ACS (18.5%); and 6,193 elective patients (56.4%). However, in the stented population were 9,886 patients consisting of 2,521 STEMI patients (25.5%); 1,867 NSTEMI-ACS patients (18.9%); and 5,498 elective patients (55.6%). There were 4,336 patients (44%) treated with DES only, hereafter called the DES patients, and 5,044 (51%) treated with passive stents only. A hybrid population consisting of 506 patients (5%) who had received both types of stents was excluded from the comparisons. The relative percentages of patients treated with a DES in the three clinical settings above were 37.1%,
46.9% and 55.1% respectively, which represented 9%, 9% and 31% of the stented patients (Figure 1).
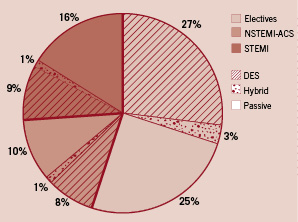
Figure 1. The stented acute and elective patients (N=9886) and the proportion of DES use in each group.
Statistical methods
Categorical data are presented as percentages or absolute numbers. For continuous variables the median, but for age the mean is shown. Subgroups were compared by Pearson chi-square test with respect to dichotomous variables, by Cochran-Armitage test with respect to ordinal categorical variables, and by Mann-Whitney U-test with respect to counts and continuous variables. A significance level of 0.05 was assumed and all p-values are the results of two-tailed tests. Adjusted odds ratios with 95%-confidence intervals were calculated by multiple logistic regression for the effect of DES vs. passive stent use on in-hospital mortality. The indication groups (STEMI and NSTEMI-ACS) and those baseline characteristics listed in table 1 which yielded p<0.2 in the univariate comparison and were chosen by a forward selection procedure with an entry level of p=0.1 were included in the model for the stented population. In a second model for the NSTEMI-ACS patients only, an age-adjustment was made due to the small number of events. The statistical computations were performed using SAS, version 9.1 (Cary, North Carolina, USA).
Results
Demographics
In the stented patient population (N=9,886) that included the hybrid population, the Mediterranean countries contributed the most patients treated with a DES (2,161 patients, 44.6%), followed by Western Europe (1,808 patients, 37.3%), Central Europe (632 patients, 13.1%) and Northern Europe (241 patients, 5.0%). Patients treated with passive stents only were predominantly from Central Europe (2,512 patients, 49.8%) followed by the Mediterranean countries (1,416 patients, 28.1%), Western Europe (1,009 patients, 20.0%) and Northern Europe (107 patients, 2.1%). The relative amount of DES used in the stented population was however highest in Northern Europe (69.3%), followed by Western Europe (64.2%), the Mediterranean countries (60.4%) and Central Europe (20.1%). The demographic characteristics of cardiac patients vary throughout Europe. Western European countries presumably have a relatively longer life expectancy (due in part to the lower age-specific mortality from cardiovascular disease) and decreasing birth rates17. In addition, the prevalence of cardiac risk factors such as smoking, obesity, diabetes, dyslipidaemia and elevated blood pressure markedly vary across countries, and is reflective of the variable distribution of healthcare resources. Realising this, we have pooled the patient demographics of stented patients across Europe (Table 1).
The data showed that almost three-quarters of the total patient population were male (74.9%), and that the median BMI was 27.1. STEMI patients were the youngest on average and had the highest number of smokers. Elective patients had significantly more cardiac risk factors and past cardiac history. The diabetics represented 24.5% of the population and they were more likely to be treated with DES. Significantly more DES was also used in elective and NSTEMI-ACS patients with dyslipidaemia. In patients admitted with a NSTEMI-ACS significantly more DES were implanted in those with previous CABG and also in patients with previous PCI regardless of their clinical presentation. There were no significant differences between the numbers of DES and passive stents implanted in patients with hypertension or renal failure.
Medication
Anti-platelet therapy on admission and discharge
On admission only 32.1% of electively stented patients were on clopidogrel (ticlopidine 9.3%) and 84.5% were on aspirin. Understandably, patients admitted with NSTEMI-ACS and STEMI were on lower amounts of clopidogrel (26.4% and 10.5%) and aspirin (66.7% and 41.1% respectively). But interestingly, more DES than passive stent treated patients in both elective (39% vs. 24.4%, p<0.001) and NSTEMI-ACS (35.1% vs. 20.0%, p<0.001) patients were admitted on clopidogrel (Table 2). In patients administered clopidogrel in-hospital, the majority (80.7%) were given the 300 mg dose. In the elective patients not admitted on clopidogrel, 38.8% of them had this drug started on the operating table.
According to the current ESC guidelines, dual anti-platelet treatment (aspirin and clopidogrel) is recommended for six months following a DES implantation and for a period of 3-4 weeks following a BMS18. But in view of the controversies regarding late stent thrombosis, the Food and Drug Administration (FDA) in the United States has extended dual anti-platelet for 12 months after treatment with a DES19. At discharge, 93.0% of electively treated DES patients and 78.4% (p<0.001) of patients treated with passive stents were on clopidogrel with 98.0% and 97.4% respectively on aspirin. Out of those DES patients who received dual anti-platelet therapy, only 23.8% were recommended clopidogrel for six months in accordance with the current ESC guidelines. The majority (69.2%) was recommended to continued treatment for at least nine months. Similarly, in elective patients treated with a passive stent, a longer period of treatment with clopidogrel was also prescribed, as 23.4% were recommended dual anti-platelet treatment for four weeks and 46.0% for at least nine months. This recommendation was equally noted in DES treated STEMI and NSTEMI-ACS patients with less than a quarter prescribed clopidogrel for six months and more than two-thirds to continue treatment for more than nine months. Prolonged treatment with clopidogrel (at least nine months) was also given to passive stent treated STEMI and NSTEMI-ACS, representing 36.6% and 46.0% of these patients respectively. Only 47.5% of STEMI and 39.7% of NSTEMI-ACS patients treated with a passive stent were recommended dual anti-platelet treatment for three months or less.
Intervention on patients treated in the three clinical settings
In the treated STEMI population, 86.1% had a primary intervention, 9.5% a rescue PCI and 4.4% a facilitated procedure. Electives had the highest amount of patients with single vessel disease (45.0%) followed by STEMI (43.3%) and NSTEMI-ACS (38.9%). Triple vessel disease was more prevalent in the NSTEMI-ACS (26.4%) and STEMI (25.0%) and less so in the elective patients (21.6%). The number of diseased vessels was significantly different between patients treated with DES and passive stents in STEMI, but not in the electives and in NSTEMI-ACS patients. The prevalence of triple vessel disease was 21.3% and 20.6% (p=0.250) in electives, 25.0% and 24.6% (p=0.776) in NSTEMI-ACS and 22.0% and 25.1% (p=0.033) in STEMI (Table 3).
Vessels diseased and treated
Angiographically, in the subgroup of patients treated with either DES or passive stents (N=9,380), the most diseased vessel (stenoses of at least 50%) was the LAD (67% in elective patients and 70% in STEMI and NSTEMI-ACS patients) followed by the RCA (54% in electives and 56% in NSTEMI-ACS with 62% in STEMI). The LCx was the least diseased vessel in all categories with 50% in electives, 54% in NSTEMI-ACS and 46% in STEMI. There were more diseased LMS and coronary bypasses in the elective (4.7% and 5.0%) and NSTEMI-ACS patients (6.4% and 4.9%) respectively. The STEMI group had the least diseased LMS (3.8%) and coronary artery grafts (0.9%). In terms of treatment (Figure 2), 36.8% of elective patients had their RCA treated corresponding to 68% of the total diseased elective RCA.
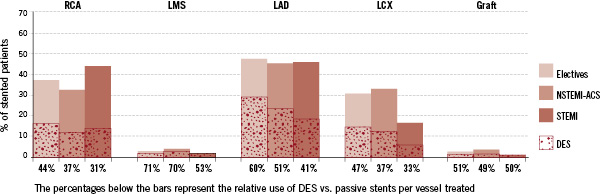
Figure 2. The relative percentage of vessels treated either with a passive stent of DES.
The RCA was treated in 32.3% of NSTEMI-ACS and 43.5% of STEMI patients representing 58% and 70% of this diseased vessel being treated accordingly. In almost half of the patients (47.8% in electives, 45.6% in NSTEMI-ACS and 45.9% in STEMI), the LAD was treated resulting in about two-thirds (71%, 65% and 65% respectively) of this diseased vessel being treated. A smaller percentage of patients had treatment to their LCx (30.7% of electives, 32.8% of NSTEMI-ACS and only 16.5% of STEMI) representing 62%, 61% and 36% of diseased LCx being treated. Only 2.5% of elective patients, 3.6% of NSTEMI-ACS and 1.2% of STEMI patients had intervention to the LMS and these represented the smallest percentages of any diseased vessel being treated (51%, 53% and 32% respectively). The amount of patients with treated coronary grafts was equally small, 2.3% in electives, 3.3% in NSTEMI-ACS and 0.6% in STEMI patients. But unlike the other vessels, the relative percentage of diseased grafts being treated seemed to increase with clinical instability from 44%, 60% to 71% in electives, NSTEMI-ACS and STEMI patients respectively.
The relative use of DES vs. passive stents per vessel treated
It is useful to know the relative amount of DES to passive stent used to treat a diseased vessel as a physician might have placed more importance on DES in certain clinical settings or vessels. As such in patients in whom RCA was treated, overall relatively less DES compared to passive was used (44% in electives, 37% in NSTEMI-ACS and only 31% in STEMI) (Figure 2). Much more importance was however placed on DES in the treatment of the LAD and LMS. In elective and NSTEMI-ACS patient populations, the relative percentage of DES used was 60% and 51% respectively for the LAD and 71% and 70% respectively for the LMS. In the STEMI patients the relative amount of DES to passive stents remained low for the LAD (41%) and the LCx (33%) although relatively more was used for the LMS (53%) and coronary grafts (50%). Similarly, lower percentages of DES were also used to treat the LCx in NSTEMI-ACS (37%), and STEMI (33%) with only 47% used in electives. Similar amounts (51%, 49% and 50%) were used to treat coronary grafts in elective, NSTEMI-ACS and STEMI patients.
Lesions treated
Amongst the 15,453 documented lesions treated with a stent or balloon angioplasty, the vast majority were type B (59.0%), followed by 27.7% type C and 13.3% type A lesions. The LAD had the most treated lesions (39.9%), then the RCA (33.7%), the LCx (23.0%), with LMS and bypass grafts each representing 1.7%. There were 5.9% in-stent restenotic (ISR) lesions and 13.4% lesions classed as bifurcations. Prior to treatment, 59.1% had TIMI 3 flow with 19.2% having TIMI 0, after treatment TIMI 3 flow was achieved in 93.7% with TIMI 0 reduced to 2.0%. Type C lesions had the least TIMI 3 flow (39.2%) and showed the maximal change, 88.3% following PCI. Direct stenting was undertaken in 43% of all stented lesions with the most used (63.7%) in type A. The use of direct stenting decreased with lesion complexity with 46.7% for type B and only 25.4% in type C.
The relative use of DES vs. passive stents per lesion treated
With regard to the choice of stent, a passive stent was implanted in 45.7%, a DES in 43.6% and no stent in 10.7% of the 15,453 treated lesions. This meant that overall 92% of type A, 90% of type B and 87% of type C significant lesions was stented. In the group of patients treated with either DES of passive stents, 12,580 lesions were stented in total. Of these stented lesions, 34% of type A and 47% of type B lesions were treated with a DES with relatively more DES (58%) used to treat type C lesions; these represented 5%, 28% and 16% respectively of all stented lesions (Figure 3).
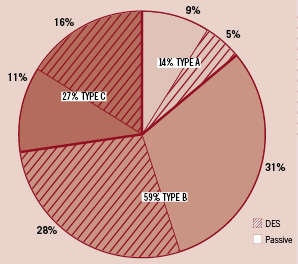
Figure 3. Relative percentage of DES in 12,580 stented lesions.
In terms of the clinical settings, type B lesions were highest amongst elective patients (59.9%) over what was noted in NSTEMI-ACS (58.4%) and STEMI (58.1%), with the relative percentage of DES used being 54%, 43% and 33% respectively (Figure 4).
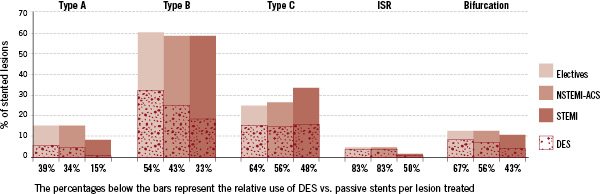
Figure 4. The relative percentage of stented lesions treated with a DES.
Stented type C lesions (24.6% in electives, 26.6% in NSTEMI-ACS and 33.4% in STEMI), had more DES used in electives (64%) and NSTEMI-ACS (56%), but less so in STEMI (48%). The tendency to treat STEMI patients with relatively less DES was also observed in other complex lesions such as bifurcations and patients with in-stent restenoses (ISR).
In elective patients, the percentage of ISR and bifurcation lesions was 5.0% and 12.9% respectively and DES usage in these lesions were 83% and 67% compared to passive stents. A similar finding was observed in NSTEMI-ACS, where ISR and bifurcations were found in 4.9% and 12.9% of the lesions and in 83% and 56% of the cases where respectively a DES was used. This was unlike STEMI patients in which there was only 2.0% ISR and 10.6% bifurcations and where they had relatively less DES usage in these lesions compared to the other groups (50% and 43% respectively). In all clinical settings, type A lesions were treated with relatively less DES, 39% in electives, 34% in NSTEMI-ACS and 15% in STEMI patients. In terms of the statistical differences between the lesions stented with DES and passive stents, significantly more passive stents were used to treat lesions in the RCA and significant more DES for those in the LAD and LMS (Table 4). More DES were used for the lesions in LCx except in the STEMI population. There was no significant difference noted in the treatment of lesions in coronary bypass grafts. Spanning lesion complexity, significant differences were noted between the two stents, with significantly more passive stents used in the simpler lesions (type A and B) and more DES in the complex ones (type C, ISR and bifurcations).
Stent/balloon diameter and DES use
The use of DES and passive stent/balloon size varied with the clinical scenario. In electives, 66.2% of the stented lesions were treated with a stent that was < 3 mm and of these 58% used a DES. In NSTEMI-ACS and STEMI stents < 3 mm were used in 65.8% and 59.3% of lesions respectively, but unlike electives there was relatively less DES used compared to passive stents (47% in NSTEMI-ACS and 34% in STEMI). Larger stents sizes (>3 mm) were used in 34% of elective, 34% of NSTEMI-ACS and 41% of STEMI. However, with stents >3 mm far less lesions were treated with a DES (40% in electives, 35% in NSTEMI-ACS and 23% in STEMI), relative to a passive stent. It therefore implied that for smaller calibre vessels lesions were more likely to be treated with a DES if they were stable and with a passive stent if they were unstable or had a larger than 3 mm calibre (Table 4).
The use of diagnostic and other therapeutic devices
In the treated patient population (N=10,982) diagnostic devices were used in only 4.1% of the cases. The use of intravascular ultrasound (IVUS) was, in general, low (1.8%) as it was only used in 1.0% STEMI, 1.1% NSTEMI-ACS and 2.4% of elective patients. Electives had the most pressure wire studies (1.7%), followed by NSTEMI-ACS (1.1%) and STEMI (0.4%). Cutting balloon (2.3%) was the most employed therapeutic device, used in 3.4% of elective patients, 1.9% of NSTEMI-ACS and 0.2% of STEMI patients. The use of intra-aortic balloon pump (IABP) was also low (1.8%) and was mostly limited to STEMI patients (4.2%) with smaller numbers used in NSTEMI-ACS (1.7%) and elective patients (0.8%). It was not surprising to find that although thrombectomy devices usage was also low (2.1%), the majority was used in STEMI patients (7.2%) with only 1.3% in NSTEMI-ACS and negligible amounts in electives (0.2%). In electives and STEMI, significantly more diagnostic devices were used in DES treated patients compared to passive stented patients (Table 5). In terms of individual diagnostic device, IVUS was used in significantly more DES treated elective and STEMI patients. Pressure wire usage was also more significant in electively treated DES patients. There were no significant differences noted in other diagnostic device used (flow wire and angioscopy) between DES and passively stented patients. In terms of therapeutic device usage, significantly more elective passively stented patients used an intra-aortic balloon pump (IABP). The reverse however was observed in NSTEMI-ACS patients, where IABP usage was slightly more in the DES treated population. In all clinical settings DES treated patients more underwent thrombectomy and there was more rotablation performed in DES treated electives.
PCI results and patients outcome
Elective patients had the least periprocedural complications (3.8%) and that this increased with clinical severity, NSTEMI-ACS (7.0%) and STEMI (13.1%). The two major complications noted were side-branch occlusion and no flow/slow flow in the vessel. Side branch occlusion occurred in 1.4% STEMI patients, 1.7% NSTEMI-ACS and 1.0% electives with no flow/slow flow occurring in 4.6%, 1.4% and 1.2% in the same patient categories. Cardiac pacing was mostly used in STEMI patients (2.5%) with relatively lower numbers of patients requiring pacing in NSTEMI-ACS (0.4%) and electives (0.1%). Tamponade, cardiogenic shock induced by the procedure and allergic reactions all occurred in less than 1% of patients within each subgroup. However, STEMI patients understandably had more cardiac arrest (2.0%) compared to NSTEMI-ACS (0.8%) and electives (0.3%). Coronary artery bypass grafts were planned in 2.0% of STEMI with 1.2% in NSTEMI-ACS and 0.7% in electives. Emergency bypass surgery was performed in 0.4% of STEMI, 0.2% of NSTEMI-ACS and 0.1% of elective patients.
In assessing the difference between stent usages it was interesting to note that only in STEMI patients were DES complications significantly less compared to passive stents (10.3% vs. 13.6% p=0.019). This may be because distal embolisation was more common in STEMI patients treated with a passive stent (2.4% vs. 0.8% p=0.008). Apart from these differences there was no other single procedural complication that was significantly more common in DES or passive stents, including stent thrombosis during the first two post-procedural days. The frequency of acute stent thrombosis was highest in STEMI (1.3%) and lower in electives (0.2%) and NSTEMI-ACS (0.1%).
In addition, post-procedural re-infarction was equally low (2.0%) occurring mostly in unstable patients (3.3% of NSTEMI-ACS and 3.2% of STEMI) and less so in the stable elective patients (1.1%) (Table 5). A significant difference in post-procedural non-fatal MI between DES and passive stented patients was only observed in NSTEMI-ACS patients. The overall in-hospital mortality in this population of treated patients in Europe (N=10,982) was 1.8%, consisting of 5.2% in STEMI, 1.8% in NSTEMI-ACS and only 0.3% in elective patients. In the stented population (N=9,380), the overall mortality was 1.7% with STEMI (4.9%), NSTEMI-ACS (1.4%) and electives (0.2%). Fifty-four patients (1.2%) in the DES treated population and 105 (2.1%) of the passive stented patients died in hospital resulting in a significant difference between the two types of stents overall (p=0.002). After adjustment for indication group, age, current smoking, history of CAD and hypertension this significance was however lost (adjusted OR=0.88, 95%-CI 0.63-1.24, p=0.47). On subgroup analysis, no significant difference in in-hospital mortality was seen in electives or STEMI patients treated with either a DES or a passive stent. But, NSTEMI-ACS patients treated with DES had marginally better in-hospital mortality (0.8% vs. 1.9% p=0.049) (Table 5). A trend was still present in the age-adjusted odds-ratio (OR=0.45 95%-CI 0.18-1.13, p=0.09). In hospital MACCE (N=10982) that took into account mortality, MI and cerebrovascular accidents (CVA) were, as expected, highest in STEMI (7.8%) and lowest in electives (1.4%) with NSTEMI-ACS having a MACCE rate of 5.4%. The MACCE reflected the results observed with MI, where a significant difference between DES and passively stented (3.0% vs. 6.4% p=0.001) was only observed in NSTEMI-ACS patients. In both STEMI (6.9% vs. 8.0% p=0.316) and elective (1.7% vs. 1.3% p=0.236) patients the MACCE rate was relatively similar.
Limitations
This data is only reflective of the participating countries and centres and does not include the total usage of stents per country. The data presented was audited, prepared and statistically analysed by the EHS. Treated lesions were not sub-categorised to a degree that complete comparison between the specific lesion and SIRIUS, TAXUS-IV and TAXUS-V was possible (reference diameter was not supplied in the database, but final balloon size had to be used as a proxy measure). This meant that guideline adherence could not be accurately assessed in patients presenting with stable or unstable angina.
Discussion
The use of DES in Europe varies markedly depending on the country and the region. Consequently, we must take into account a number of social and economic factors related to a country’s healthcare when assessing the presented data. It is not surprising therefore that passive stent usage predominates in Central Europe, however, notably in this study, both Western and Northern European countries together used less DES than the Mediterranean countries. This may be because the current data biases countries that were more prone to collect and submit clinical details and so may not be truly representative across all borders. However, more precise information will ultimately be available through the PCI registry databases as member states and their hospitals continue to submit their patient’s information. Nevertheless, the data currently shows that there is more passive stent to DES use in the selective acute and elective European patients during the period June 2005 to December 2005. The fact that DES were used predominately in electively treated patients and least in STEMI patients is in accordance with the current ESC guidelines12, as the trials which formed the basis of these guidelines excluded patients with acute infarcts. This may have been due to the perception that DES use in STEMI was thought to be more thrombogenic and “less endothelial friendly”20. Also, in STEMI patients, the recurrence of angina due to restenosis is low because of the already damaged myocardium, and therefore the number of target lesion and target vessel revascularisation (TLR/TVR) will be naturally reduced, thus favouring passive stent usage. The fact that the EHS results showed that in STEMI there was no over-representation of acute stent thrombosis in DES over passively stented patients may add to this debate. Acute thrombosis is more often associated with a mechanical problem, such as the stent malapposition or a vessel dissection rather than to the characteristic of the drug21. However, in the current climate, where reportedly there is 0.6% late stent thrombosis in DES per annum, the long-term follow-up of all patients regardless of their clinical presentation will be crucial in defining the future role of DES therapy9. Two recent randomised trials have compared the use of DES and BMS in STEMI. In the PASSION trial there was no difference between the paclitaxel-eluting stent (PES) and BMS in the primary composition end point of death from cardiac causes, re-infarction requiring hospitalisation, and ischaemia-driven TLR within 12 months22. A similar finding was observed in the TYPHOON trial addressing sirolimus eluting stent (SES) and BMS20. Although there was a significantly lower rate of target-vessel failure (TVF) observed at one year (7.3% vs. 14.3%, p=0.004), this difference was driven by a significant reduction in the rate of TVR in the SES group, as compared with the BMS group (5.6% vs. 13.4%, P<0.001) as the rates of death (2.3% in the sirolimus-stent group and 2.2% in the BMS group) and re-infarction (1.1% and 1.4%, respectively) were almost identical20. In both studies, one late stent thrombosis was reported and the data from these two trials suggested that DES can be used safely in the setting of primary PCI and are likely to reduce the need for repeated revascularisation.
Interestingly, more DES were used in patients with previous PCI presumably because it was thought that these patients were more likely to have progression of their atherosclerotic disease in the future and hence deserved a more “robust” treatment. There is however growing evidence to support DES in the treatment of ISR23,24 and this was reflected by its high usage in elective and NSTEMI-ACS restenotic cases (79% and 80%) with a lesser amount in STEMI (57%). Uncontrolled trials comparing sirolimus and paclitaxel stents showed reduction in restenotic rates25. But it is also important to realise that the majority of stents used to treat ISR were under-deployed after their initial implantation and that significant gains can be achieved following high-pressure balloon dilatation and IVUS guidance26,27. Despite this the use of diagnostic devices including IVUS to aid stent implantation for ISR in Europe was only 6.9%.
In accordance with current guidelines the larger vessels of 3.0-4.0 mm in diameter were more significantly treated with a passive stent whilst in vessels less than 3.0 mm a DES was used preferentially. There was a tendency to treat more LAD and LMS with DES, probably because of their adjudged greater importance, unlike the RCA where passive stents were more often used. This was also illustrated by the use of more passive stents for type A lesions. However, as lesion complexity increased preference for a DES also increased, as exemplified by LMS, ISR and bifurcation lesions across all clinical scenarios. But, the use of DES in this setting is at an evidence rating IIc and recent published data on SES in bifurcations emphasised the persistent limitations related to the routine stenting of the side branch28. Also the stenting of the main branch with balloon dilatation for the side-branch compared to stenting for both branches showed no statistically significant differences between the two strategies29. With two or even three layers of struts of DES apposed to the vessel wall (as with the crush technique) there is the possibility of increased thrombogenicity and the associated delayed endothelialisation may increase the risk of thrombosis beyond 30 days.
Diabetic patients accounted for more than a quarter of the patients with almost 60% of elective diabetics being treated with DES. The view that DES may reduce the restenotic rate and complications in diabetics may be somewhat premature as data from the RESEARCH and T-SEARCH registries have revealed that PES, but not SES, was superior to BMS in reducing major adverse cardiac events and after propensity analyses, none of the differences remained significant30. Also, the incidence of stent thrombosis (ST) was high in both DES groups at two years follow-up accounting for 4.4% of the SES patients (3.4% early ST) compared with 2.4% in the PES group (2.0% early ST) and only 0.8% in the BMS group (0.8% early ST). The e-Cypher registry also had over a quarter of diabetics and showed that insulin-dependent diabetes was a clinical predictor of stent thrombosis with SES31. It is therefore not surprising that in the current climate of stent thrombosis, prolonged use of dual antiplatelet therapy is recommended and is sanctioned by the FDA10. At 12 months about 60% of DES, and over one third of passively stented patients, were still on clopidogrel. Prolongation of treatment is probably reasonable as an observational study from the Duke Heart Center on patients having clopidogrel for 12 months had significantly lower rates of death or MI at 24 months compared to patients not on dual anti-platelet treatment32. However, amongst BMS treated patients the continuation of clopidogrel therapy for 12 months showed no difference in death and MI. It was therefore reassuring to observe that only 1.6% of DES treated patients in the EHS had clopidogrel for a month or less as recent data from a 19-centre study of MI-patients revealed that patients who stopped thienopyridine therapy by 30 days were significantly more likely to die during the next 11 months or to be re-hospitalised33.
Percutaneous intervention in Europe has low overall in-hospital mortality of 1.8% with understandably STEMI patients most at risk (5.2%), least in electives (0.3%) and 1.8% in NSTEMI-ACS. We do not have long-term follow-up data on the EHS but we do have 3-year data on DES use in STEMI and electives from the T-SEARCH and RESEARCH registries. They showed that in electives there is no difference in MACCE between PES and SES, and favours DES over BMS, but in STEMI the initial advantage DES over BMS is now being reduced34.
Conclusion
Overall more complicated lesions were treated with DES than lesions used in the clinical trials for the current DES guidelines. The importance of this is that the off-label use of DES in complex lesions may create a bias in long-term outcome analysis due to the higher risk of restenosis and thrombosis. Adherence to the guidelines was not absolute in terms of dual platelet inhibitor treatment in view of the attention drawn to late stent thrombosis. Patients with diabetes or prior PCI, with their higher likelihood of restenosis, were significantly more often treated with DES. Importantly, coronary intervention with DES in Europe carried lower periprocedural complications and in-hospital mortality, but there was no over representation of acute stent thrombosis over passive stents even in STEMI treated patients. Follow-up of these patients is eagerly awaited to further refine the current guidelines.
Appendix
Organisation of the Survey
Statistical analysis centre (Institut für Herzinfarktforschung, Ludwigshafen, Germany): M. Hochadel (Statistician).
National Coordinators: Kurt Huber, Austria; Guy De Backer, Belgium; Vera Sirakova, Bulgaria; Roman Cerbak, Czech Republic; Per Thayssen, Denmark; Osama Abdel Aziz, Khalid Tammam, Egypt; Seppo Lehto, Finland; François Delahaye, France; Bondo Kobulia, Georgia; Uwe Zeymer, Germany; Dennis Cokkinos, Dimitrios Kremastinos, Greece; Kristof Karlocai, Hungary; Emer Shelley, Ireland; Shlomo Behar, Israel; Aldo Maggioni, Italy; Virginija Grabauskiene, Lithuania; Jaap Deckers, Netherlands; Inger Asmussen, Norway; Janina Stepinska, Poland; Lino Gonçalves, Candida Fonseca, Portugal; Vyacheslav Mareev, Russian Federation; Zorana Vasilijevic, Serbia & Montenegro; Igor Riecansky, Slovakia; Miran F. Kenda, Slovenia; Jose Luis Lopez-Sendon, Spain; Annika Rosengren, Sweden; Peter Buser, Switzerland; Tugrul Okay, Turkey; Oleg Sychov, Ukraine; Peter Schofield, United Kingdom.
There was no national coordinator in participating countries which are not mentioned in the above list.
EuroHeart Survey Board Committee: A.K. Gitt (chairman), Germany; L. Tavazzi, Italy; R. Seabra Gomes, Portugal; J. Marrugat de la Iglesia, Spain; L. Wallentin, Sweden; P. Kearney, Ireland; K. McGregor, France; M.L. Simoons, The Netherlands.
Industry Sponsor: Main sponsors: Boston Scientific, GlaxoSmithKline. Sponsors: Bristol-Myers Squibb, Eli Lilly.
List of Institutions: Belgium Working Group of Interventional Cardiology, Czech Society of Cardiology, Portuguese Cardiac Society,
Participating Centres, Investigators and Data Collection Officers:
Armenia: Kristine Margaryan, Shahen Khachatryan, Yerevan; Austria: Franz Weidinger, Jakob Doerler, Eva-Maria Stocker, Innsbruck; Johann Altenberger, Matthias Heigert, Max Pichler, Salzburg; Gunther Christ, Helmut Glogar, Irene Lang, Stefan Ingerle, Vienna; Belgium: Philip De Wilde, Michel de Marneffe, Bruxelles; M. Vrolix, Joseph Dens, Johan Van Lierde, Genk; X. De Wagter, Gent; Marc Carlier, Gilly; Antoon Weyne, Kortrijk; Victor Legrand, Pierre Doneux, Olivier Gach, Laurent Davin, Liège; Eric Mievis, Pierre-Emmanuel Massart, Namur; G. Holvoet, Oostende; Croatia: Lovel Giunio, Duska Glavas, Ivica Vukovic, Branimir Markovic, Darko Duplancic, F. Runjic, Spilt; Edvard Galic, Jure Mirat, Zagreb; Czech Republic: Petr Kala, Jiri Semenka, Ota Hlinomaz, Erik Petrikovits, Brno; Petr Widimsky, Petr Tousek, Prague; Ivo Varvarovsky, Pardubice; Denmark: P. Thayssen, Helle Cappelen, Odense; Steffen Helqvist, Henning Kelbaek, Erik Jorgensen, T. Engstrom, Kari Saunamaki, Jens Kastrup, Peter Clemmensen, H. Hansen, Copenhagen; Egypt: Magid Al Abbadi, Khalid Tammam, Hany Abdel Razek, Gamal Aboul el Nasr, Hany Ragi, Basam Ibrihim, Basam Zarif, Naha el Banhawy, Khalid Sorour, Mohamed Abdel Meguid, Ahmed Mahrous, Cairo; Khalid Ahmed Al Khashab, Fayoum; Ahmed Abd Elmoniem, Metwaly El Emry, Amr El Naggar, Benha; Aly Saad, Zagazig; Estonia: Peep Laanmets, Jyri Voitk, Piia Lutter, Sigrid Jarvekulg, Mati Jalakas, Julia Reinmets, Toomas Marandi, Margus Peeba, Tarmo Serka, Tallin; Finland: Mikko Syvannne, Esa Kaihovirta, Helsinki; Kari Korpilahti, Mari-Anne Vaittinen, Vaasa; France: Jean-Pierre Bassand, Denis Pales Espinosa, Besancon; Yves Cottin, Isabelle Lhuillier, Philippe Buffet, L. Lorgis, Dijon; Jacques Machecourt, Bernard Bertrand, Delphine Serrano, Grenoble; Jean-Louis Bonnet, Marseille; Philippe Gabriel Steg, Jean-Michel Juliard, Reza Farnoud, Paris; Nicolas Delarche, Pau; Jean Marco, Frederic Petit, Bruno Farah, Didier Carrie, Michel Galinier, Jacques Puel, Jacqueline Cahuzac, Jerome Roncalli, Stephane Tauzin, Meyer Elbaz, Toulouse; Germany: Volker Schachinger, Frankfurt; Anselm Gitt, Uwe Zeymer, Ludwigshafen am Rhein; Ralf Zahn, Boris Fraiture, Siegberto Haetinger, Nurnberg; H. Klepzig, E. Girth, A. Hauber, Offenbach; Christian Firschke, Jochen Widmaier, Florian Hofbauer, Stefan Huttl, Pfaffenhofen; Udo Sechtem, Ulli Parade, Stuttgart; Georg Linnartz, Viersen; Greece: D. Cokkinos, Stalikas, Andrianidis, Natassa Tsiavou, Athens; Georgios Papaioannou, Efthymios Deliargyris, Maroussi Attikis; Dimitrios Alexopoulos, Periklis Davlouros, Patras; Dimitrios Tsikaderis, Petros Dardas, Nikolaos Mezilis, Thessaloniki; Hungary: Edes Istvan, Bakos Zoltan, Debrecen; Israel: Yoav Turgeman, Suleiman Khaled, Alexander Feldman, Afula; Jamal Jafari, Iliya Manevich, Ashkelon; Carlos Cafri, Reuben Ilia, Akram Abu-Ful, Sergei Yaroslavslev, Jean Mark Wainstain, Gabriel Rosenchtein, Beer Sheva; Ricardo Krakover, Beer Yakov; David Halon (& the CV Medecine department), Luis Gruberg, Walter Markiewicz, Ehud Grenadier, Monther Boulos, Ariel Roguin, Arthur Kerner, Shlomo Amikam, Margalit Ben-Tzvi, Jeremy Rezmovitz, Haifa; Morris Mosseri, Haim Lotan, Boris Varshizky, Hisham Nassar, Haim Daninberg, David Rot, Tedi Vais, Jesaia Benhorin, Andre Keren, Aharon Medina, Zahi Huri, Jerusalem; Simcha Brandis, Guy Schoenmann, Netanya; Ran Kornowski, Abed Assali, Shmuel Fuch, David Hasdai, David Brosh, Rehavia, Offer Sela, Igal Teplitski, Petach Tikva; Oded Eisenberg, Rehovot; Shmuel Banai, Ariel Finkelstein, Tel-Aviv; Yonathan Hasin, Muein Aboud, Menachem Nahir, Daud Qarwani, Genem Diab, Tiberias; Italy: Luigi Meloni, Giorgio Lai, M. Cadeddu, R.Pirisi, Cagliari; Francesco Bonechi, Franco Nassi, Massimiliano Nieri, Andrea Taiti, Alessandra Naldoni, Francesco Calabro, Fucecchio; Felice Achilli, Stefano Maggiolini, Luigi Piatti, Gianluca Tiberti, Piero Addamiano, Lecco; Sergio Berti, Marcello Ravani, Cataldo Palmieri, Giuseppe Trianni, Simona Cardullo, Massa; Angelo Cioppa, Paolo Rubino, Vittorio Ambrosini, L. Salemme, G. Sorropago, T. Tesorio, Mercogliano; Giuseppe Geraci, Modena; Filippo Scalise, Simone Mazzeti, Carla Auguadro, Monza; Giovanni Esposito, Napoli; Guido Canali, Negrar; Maria Enrica Caccia, Chiara Ruggieri, Bertola Benedetta, Novara; Nicoletta de Cesare, M. De Benedictis, Tiziana Coco, Sabrina Manzotti, Osio Sotto Fraz. Zingonia; Paolo Marraccini, Pisa; Alessandro Danesi, R. Ricci, A. Ferraironi, E. Olivieri, A., Chiera, Roma; Stefano Garducci, Daniela Grasseli, Vimercate; Ireland: Peter Kearney, Eugene McFadden, Noel Cahill, Cork; Martin Quinn, Peter Crean, Eileen Caroll, David Foley, Stephen O’Connor, Rory O’Hanlon, Bernadette Lynch, Sarah O’Donnell, James Roy, Darragh O’Brien, Dublin; Latvia: Asnate Krastina, Andrejs Erglis, Riga; Lebanon: Samih Lawand, Beirut; Poland: Waldemar Dorniak, Jacek Klaudel, Krzysztof Pawlowski, Wojciech Trenkner, Gdansk; Marianna Janion, Marcin Sadowski, Agnieszka Janion-Sadowska, Kielce; Izabella Skorupa, Leszek Bystryk, Lublin; Adam Kern, Olsztyn; Bartlomiej Janiak, Roman Szelemej, Walbrzych; Witold Ruzyllo, Adam Witkowski, Tomasz Deptuch, Renata Maczynska-Mazuruk, Warsaw; Andrezj Budaj, Kokowicz Lewandowski Cegieska, Grzegorz Opolski, Joanna Wilczyska, Marek Roik, Janusz Kochman, Warszawa; Portugal: Dinis Martins, Acores; Isabel Maria Fernandes Joao Goncalves, Helder Pereira, Almada; Henrique Faria, Joao Calisto, Lino Goncalves, Victor Matos, Antonio Leitao-Marques, M. Costa, H. Oliveira, P. Mota, Coimbra; Walter Santos, Victor Brandao, Faro; Graca Caires, Bruno Silva, Funchal; Rui Campante Teles, Manuel Almeida, Pedro Goncalves, Luis Raposo, Luis Mourao, Luis Bernardes, Paulo G. Pedro, Rui Ferreira, Rui Conduto, Jorge Quininha, Lino Patricio, Duarte Cacela, Jose Maria Goncalves, Lidia de Sousa, Manuela Adao, Lisbon; Henrique Cyrne Carvalho, Helia Romeira, Jose Paulino Sousa, Jose Manuel Mota Garcia, J. Carlos Silva, Domingos Magalhaes, Porto; Ricardo Santos, Setubal; Pedro Gama Mendes, Joao Pipa, Luis Nunes, Pedro Ferreira, Viseu; Romania: Dragos Vinereanu, Cristian Udroiu, Nicolae Florescu, Octavian Parvu, Claudiu Stoicescu, Maria Dorobantu, Serban Mihai Balanescu, Rodica Niculescu, Lucian Calmac, M. Marinescu, Bucharest; Dan Olinic, Mihai Ober, Calin Homorodean, Claudia Budurea, Adrian Hij, Florin Anton, Cluj-Napoca; Florin Ortan, Ciprian Suciu, Mihai Ursu, Costica Baba, Targu-Mures; Stefan Iosif Dragulescu, Lucian Petrescu, Milovan Slovenski, Dan Gavrilescu, Cristian Dina, Bogdan Mut, Timisoara; Serbia & Montenegro: Rade Babic, Mirko Colic, Dragan Topic, Belgrad; Spain: Joan Bassaganyas Vilarrasa, Marti Puigfel Pont, Rafael Masia Martorell, Izabella Rohlfs, Girona; Rafael Melgares Moreno, Granada; Maria Irurita, Juncal Irurita, Las Palmas de Gran Canaria; Carlos Escobar Cervantes, Madrid; Teresa Galvan, Jimenez Navarro, Dominguez Franco, Malaga; Ignacio Santos Rodriguez, Victor Hogo Ramirez, Salamanca; Francisco Fernandes-Aviles, Ana Revilla, Pedro Mota, Valladolid; Switzerland: Nicholas Masson, Virginie Dupertuis, Genève; Tunisia: Salem Kachboura, Ariana; Turkey: Atila Iyisoy, Ankara; Mustafa Kemal Erol, Erzurum; Zeki Ongen, Erhan Babalik, Mehmet Oskan, Nihal Ozdemir, Ali Oto, Kudret Aytemir, Bunyemin Yavuz, Istanbul; Mahmut Sahin, Kenan Durna, Samsun; Vedat Aytekin, Cemsit Demiroglu, Murat Gulbaran, Saide Aytekin, Alp Burak Catakoglu, Burak Ozme, Gokmen Gemici, Hasan Feray, Sisli; United Kingdom: P.M. Schofield, S. Kahn, S. Clarke, Heather Millington, Cambridge; Carlo Di Mario, Debra Dempster, London; Robert Anthony Henderson, Jane Burton, Della Falcon-Lang, Nottingham.

