Abstract
Background: Randomised studies have demonstrated the beneficial effect of drug-eluting stents in reducing repeat revascularisation at one year. However, they were individually underpowered to assess long-term safety endpoints such as death and myocardial infarction. The long-term safety of drug-eluting stents has been recently questioned.
Methods and results: We performed a pooled analysis of 2,797 patients included in four randomised trials to assess the safety of slow-release paclitaxel-eluting stents as compared with bare metal stents. Patient level data were obtained and analysed by two independent academic statistical institutions. The primary safety endpoint was survival at four years. Secondary endpoints were myocardial infarction and stent thrombosis. Heterogeneities in treatment effect were tested in subgroups.
Survival at four years was 93.4% in the paclitaxel-eluting stent group versus 93.0% in the bare-metal stent group (Hazard ratio for survival 0.95; 95% confidence interval [CI] 0.96 - 1.30; P=0.75). Myocardial infarction occurred at a similar rate between both treatment groups. Whereas the total rates of stent thrombosis were equal between the two groups, there was a trend towards a higher rate of stent thrombosis occurring after repeat target lesion revascularisation in the bare-metal stent group. No heterogeneity of treatment effect was found in the subgroups, including diabetic patients and complex lesions.
Conclusions: In a pooled analysis of four trials comparing paclitaxel-eluting stents with bare metal stents, no significant differences were found in the rates of death, myocardial infarction or stent thrombosis.
Introduction
Pivotal randomised clinical trials and registries have shown that drug-eluting stents reduce the need for subsequent revascularisation procedures when compared with bare metal stents1-10. More than four million patients have been treated worldwide with drug-eluting stents. However, the pivotal randomised trials were individually underpowered to detect differences in death and myocardial infarction. Recently, several meta-analyses of published randomised trials with three to four years of follow-up have shown a small, albeit consistent increase in the rates of death, myocardial infarction and late stent thrombosis in patients receiving drug-eluting stents11-13. To specifically address the long-term safety of the paclitaxel-eluting stent, we performed a safety meta-analysis of patient level data derived from four randomised controlled trials comparing a slow-release paclitaxel-eluting stent with a bare-metal stent during a follow-up period of four years in 2,797 patients.
Methods
The original studies
The present analysis is based on pooled patient level data from the TAXUS I, II, IV, and V trials, all comparing a polymer-based, slow-release paclitaxel-eluting stent to a bare metal stent in lesions in native coronary arteries using a double-blind study design with a 1:1 randomisation process. The design of these trials, as well as short-term angiographic and clinical outcomes, have been reported previously7-10,14. Patients treated with the moderate-release paclitaxel-eluting stent, which was never commercialised, were excluded from the present analysis. For this reason, patients treated with the moderate-release paclitaxel-eluting stent and their control group of cohort II of TAXUS II and patients included in TAXUS VI were excluded. Acute myocardial infarction was an exclusion criteria in all trials. A total of 715 patients with diabetes (defined as patients which were medically treated and/or insulin treated) were included.
Angiographic inclusion criteria were variable: TAXUS I, II and IV included patients with low risk lesions whereas TAXUS V included prespecified subgroups of patients with lesions in small vessels (< 2.25 mm), long lesions (> 26 mm), and lesions treated with large diameter stents. In both TAXUS IV and V, randomisation of diabetics was stratified by group. TAXUS I and II compared the paclitaxel TAXUS NIR™ stent to the bare metal NIR™ stent and TAXUS IV and V compared the TAXUS Express™ stent to a bare metal Express™ stent (all from Boston Scientific, Natick, Mass, USA). The primary endpoints for TAXUS I as a Phase I feasibility study was 30-day MACE (a composite endpoint of all-cause death, q-WAVE myocardial infarction and stent thrombosis at 30 days). The primary endpoint for TAXUS II as a Phase II study was % in-stent net volume obstruction measured by intravascular ultrasound at six months. TAXUS IV and V as Phase III and Phase IIIb studies were powered for nine-month target vessel revascularisation as primary endpoints. Cardiac death and myocardial infarction were collected as secondary endpoints in all studies. All-cause death and stent thrombosis by protocol definition were also collected in all studies.
Dual antiplatelet therapy with aspirin and clopidogrel or ticlopidine was mandated per protocol for a minimum of six months in all studies. Aspirin was mandated indefinitely. Aspirin doses ranged from 81 to 325 mg daily.
The protocol called for angiographic follow-up at six months (for all patients in TAXUS I and II), at nine months for all patients in TAXUS V and for a prespecified subgroup of patients in TAXUS IV, and clinical follow-up data up to five years was prespecified. Clinical status was checked yearly and five year follow-up is currently available for TAXUS I, four-year for TAXUS II and IV and Two years for TAXUS V. Median follow-up was 1,430 days (IQR 1,072 to 1,452) The study protocols were approved by the ethics committee at each participating institution and were conducted according to the principles of the Declaration of Helsinki. All patients gave written informed consent before enrolment. The studies were performed between October 2000 and December 2002.
Current analysis
All studies were sponsored and monitored by the Boston Scientific Corporation. Follow-up was performed by the investigating centres in a blinded fashion. Core lab analysis for angiographic and intravascular ultrasound was performed by independent research organisations (Cardialysis, Rotterdam, The Netherlands, for the TAXUS II trial, and by Harvard Clinical Research Institute, Boston, MA, for the TAXUS I, IV and V trials). Independent clinical event committees adjudicated the events. For this specific post-hoc analysis, the patient level based data were pooled and than analysed by two independent academic statisticians. The authors had unrestricted access to the data and made all decisions about publication independently of the sponsor or the principal investigators of the studies.
Study endpoints
The primary safety endpoint was all cause death. Secondary safety endpoints were cardiovascular and non-cardiovascular death, stent thrombosis, and all cause death or Q-wave myocardial infarction and all cause death or all myocardial infarction. The same definitions of events given below were used for all four trials.
Cardiovascular death was defined as death due to any of the following: 1) acute myocardial infarction, 2) cardiac perforation or pericardial tamponade, 3) arrhythmia or conduction abnormality, 4) cerebrovascular accident within 30 days or related to the procedure, 5) complication of the procedure or 6) any death in which a cardiac cause cannot be excluded. Non-cardiovascular death was defined as any death not due to a cardiovascular cause.
Q-wave myocardial infarction was defined as the development of new, pathological Q-waves in two or more contiguous leads as assessed by the ECG core laboratory with creatine kinase (CK) or CKMB levels elevated above the upper limit of normal. Non-Q-wave myocardial infarction was defined as an elevation of CK two times the normal value with positive CKMB in the absence of new pathological Q-waves.
In the study protocols stent thrombosis was defined as an acute coronary syndrome with angiographic documentation of either vessel occlusion or thrombus within or adjacent to a previously successfully stented vessel or, in the absence of angiographic confirmation, either acute myocardial infarction in the distribution of the treated vessel or death from cardiac causes within 30 days. A thrombotic occlusion subsequent to a repeat target lesion intervention did not quality as stent thrombosis in these definitions.
Stent thrombosis was re-classified in a blinded fashion by an independent research organisation (Harvard Clinical Research Institute, Boston, MA, USA) according to a set of definitions developed during the summer of 2006 by a consortium of academic investigators, regulators and industry representatives. These definitions (referred to as the Academic Research Consortium or ARC definitions) were proposed to serve as standard criteria for stent thrombosis for the comparison of event rates across different trials and studies. Using the ARC definitions, stent thrombosis was classified as acute if it occurred within 24 hours after the index procedure, subacute if it occurred between one and 30 days, late between 30 days and one year and very late after one year. Furthermore, stent thrombosis was considered definite if there was angiographic proof of thrombus with or without vessel occlusion associated with clinical or electrocardiographic signs of acute ischaemia, or a rise of CK twice the normal value within the 48 hours of the angiogram. Stent thrombosis was classified as probable if unexplained death occurred within 30 days of the index procedure or if a myocardial infarction, irrespective of the time after the index procedure, was documented in the territory of the implanted stent without angiographic confirmation of stent thrombosis. Stent thrombosis was classified as possible in the presence of any unexplained death occurring after 30 days of the index procedure. Stent thrombotic events occurring after the index procedures were classified as primary stent thrombosis and events occurring after repeat target lesion revascularisations were adjudicated as secondary stent thrombosis.
Statistical analysis
All analyses were based on the intention-to-treat principle. Summary statistics for continuous variables are presented as medians and interquartile ranges. Categorical data are summarised as frequencies and percentages. Differences in baseline characteristics between patients randomised to paclitaxel-eluting stents versus bare metal stents were analysed using Wilcoxon-Mann-Whitney tests or Fisher’s exact tests, as appropriate.
The incidence of events over time was with the use of the Kaplan-Meier method, whereas log-rank tests and Cox proportional-hazards regression analyses were applied to evaluate differences between the two groups. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. In the main analysis, hazard ratios and 95% confidence intervals (CIs) were adjusted for differences in outcome between trials. Owing to the differences in the follow-up per study with a mean of 1,762, 1,384, 1,345 and 690 days in TAXUS I, II, IV and V respectively we decided to count events through 1,440 days for TAXUS-I, II and IV and through 730 days for TAXUS-V. Finally, two-year follow-up was available for 95.2% of the patients in TAXUS-V, and four-year follow-up was available for 100%, 96.1% and 94.1% in the TAXUS-I, II and IV respectively. No events occurred beyond 1,440 days.
Exploratory analyses (not prespecified) were performed to evaluate possible heterogeneities in treatment effects according to the trial-of-enrolment and the following clinically and angiographic relevant characteristics: age, gender, diabetes, dyslipidaemia, hypertension, prior MI, clinical presentation, diseased vessel, left ventricular ejection fraction, AHA/ACC lesion type and number of implanted stents. Treatment effects were evaluated by Cox’ PH regressions that included a characteristic times allocated treatment interaction term, with adjustment for between-trial outcome differences. More extensive regression models were applied to estimate adjusted treatment effects15.
All statistical tests were two-sided, without correction for multiple testing. P values of less than 0.05 and less than 0.01 were considered to indicate statistical significance for the results of non-heterogeneity tests and tests for heterogeneity in treatment effect, respectively. All statistical analyses were performed with the use of SAS software, version 8.2 (SAS Institute).
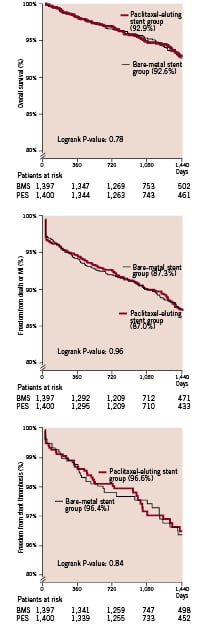
Figure 1. Kaplan Meier survival curves for patients who received a paclitaxel-eluting stent and those who received a bare-metal stent. Survival rates at 1,440 days are shown in parenthesis. P-values were calculated with the use of the log-rank test.
Results
A total of 2,797 patients were included in this analysis (TAXUS I: 61 patients, TAXUS II cohort I comparing the slow release formulation and its respective control: 266, TAXUS IV: 1,314, TAXUS V: 1,156). In total, 1,400 patients received a paclitaxel-eluting stent, and 1,397 patients were treated with a bare metal stent. The clinical and angiographic characteristics of the study patients are summarised in Table 1. Except for silent ischaemia at presentation, which was slightly more frequent in the bare metal stent group as compared with the paclitaxel-eluting stent group (18.4% versus 14.9%; P=0.015), there were no significant differences in baseline clinical and procedural characteristics between both treatment groups.
Results for all patients are shown in Table 2. The four-year cumulative survival rate was similar in the paclitaxel-eluting stent group as compared to the bare metal stent group (92.9% vs. 92.6% respectively; hazard ratio for survival for the paclitaxel-eluting stent group 0.95, 95% CI 0.70 -1.31; p=0.77). Subsequently, there were no differences in both cardiovascular and non-cardiovascular mortality. There were no differences in the occurrence of myocardial infarction and total stent thrombosis between the two treatment groups. Using the protocol definitions, stent thrombosis occurred in 16 patients in the paclitaxel-eluting stent group and 10 patients in the bare metal stent group (Table 2). When the ARC definitions were applied, stent thrombosis (either definite, probable or possible) was noted in 39 patients in the paclitaxel-eluting stent group and 41 patients in the bare metal stent group. Events occurring after repeat revascularisation for restenosis accounted for stent thrombosis in three patients with paclitaxel-eluting stents and eight patients with bare-metal stents. Of note, all these events occurred after 30 days. In the bare-metal stent group five definite or probable stent thrombotic events occurred following repeat target lesion intervention versus one on the paclitaxel-eluting stent group. Worth mentioning was that in four out of five cases brachytherapy was used whereas a new bare-metal stent was implanted in only one case. In the sole event occurring post repeat intervention in the paclitaxel-eluting stent group, brachytherapy was used as well.
No significant heterogeneity in the treatment effects was observed in any of the prespecified characteristics, including complex lesions and diabetes (Figure 2).
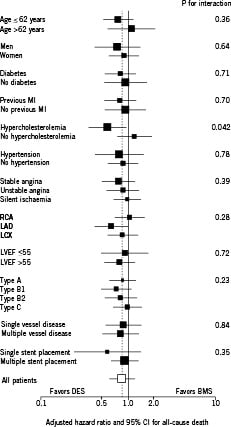
Figure 2. All-cause mortality in selected subgroups. Exploratory analyses to evaluate possible heterogeneity in treatment effects on mortality according to the trial of enrolment and the following clinically relevant characteristics: age, gender, diabetes, dyslipidaemia, hypertension, prior MI, clinical presentation, diseased vessel, left ventricular ejection fraction, AHA/ACC lesion type and number of implanted stents. The size of the squares corresponds to the amount of statistical information. For the continuous variables (age and left ventricular ejection fraction), medians were used as cut-off. Results of tests for heterogeneity in treatment effect were considered significant if P was <0.01. MI=myocardial infarction, LVEF=left ventricular ejection fraction, RVD=reference vessel diameter.
While in the overall population diabetics had a worse overall survival as compared with the non-diabetic patients, overall survival in both the bare-metal stent group and paclitaxel-eluting stent group was comparable in both the diabetic and non-diabetic subset (hazard ratio for survival for paclitaxel-eluting stent treated diabetics 0.82, 95% CI 0.54-1.23; P for interaction 0.71) (Figure 3).
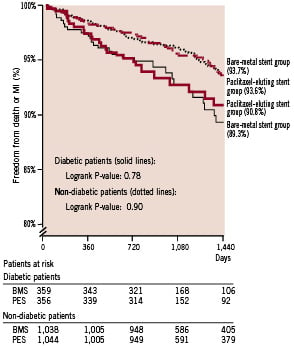
Figure 3. Kaplan Meier curve showing freedom from stent thrombosis according to the Dublin definitions. Survival rates at 1,440 days are shown in parenthesis. P-values were calculated with the use of the log-rank test.
Additionally, in the diabetic patients stent thrombosis occurred at an identical rate between the diabetic patients receiving a bare-metal stent and a paclitaxel-eluting stent (11 versus 13 events respectively according to the ARC definitions).
Discussion
In this pooled analysis of four randomised trials comparing paclitaxel-eluting stents to bare metal stents in 2,797 patients with four years of follow-up, we found similar rates of death, myocardial infarction and stent thrombosis in both groups. No heterogeneity of the treatment effect was found in the higher risk subsets, such as diabetics or patients treated for complex lesions.
Recent concerns were raised about the long-term safety of drug-eluting stents. Meta-analysis using published data and registries suggested that their use would lead to marked increases in death, myocardial infarction, stent thrombosis and even cancer. Shortly after, physician derived independent patient level based meta-analysis of randomised trials tempered these premature contentions and a dedicated two-day Food and Drug Administration panel meeting undermined the concept that the hypothesised safety concerns outweighed the benefits of drug-eluting stents for on-label use.16,17 The panelists’ opinions were more divided, and left open the possibility that death and myocardial infarction due to stent thrombosis might be increased in patients with off-label drug eluting stent use.
In this analysis, angiographically proven stent thrombosis occurred at a yearly rate of 0.2%. Similar results were noted in a pooled analysis of randomised trials, which assessed the sirolimus-eluting stent17. In contrast, the rate was 0.6% per year in a two-centre all-comers registry with a three-year follow-up.18 Careful selection and follow-up of patients in randomised trials may account for this difference. Primary angioplasty for acute myocardial infarction, stent length, diabetes, bifurcation treatment and premature antiplatelet discontinuation proved to be the strongest predictors of stent thrombosis following drug-eluting stent implantation6,18-25. Despite the inclusion of more complex patients in the more recent TAXUS studies, these characteristics were absent or less frequently observed in the present analysis. Therefore, extrapolation of our findings to a real world patient population might be far-fetched.
To better define the relative contribution of stent thrombosis to the mortality rate in both groups, the events were adjudicated according to definitions recently developed by a consensus group. Classification in three categories (definite, probable, and possible) allows uniform description and reliable comparison of stent thrombosis rates between studies and registries. Late events such as unexplained death, which were not considered in the initial protocol definitions, were adjudicated as possible stent thrombosis. Thrombotic occlusion occurring after repeat revascularisation were also adjudicated as thrombosis of the original stent. In the bare metal stent group, 28% (5/18) of the definite or probable stent thrombosis occurred following a target lesion revascularisation compared with 5% (1/22) in the paclitaxel-eluting stent group. The validity of adjudicating events occurring after repeat revascularisation to stent thrombosis of the original stent is debatable, and it is worth mentioning that a thrombotic occlusion following a repeat target lesion intervention was not considered a stent thrombosis in the initial per protocol definitions. However, stent thrombosis following repeat revascularisation emphasises the potentially severe consequences of restenosis, which is still often considered as a purely benign process. Dedicated studies of hospitalisation for in-stent restenosis showed that up to 10% of these patients presented with a myocardial infarction or sudden death26,27. Additionally, two studies demonstrated a close correlation between the rate of restenosis and late mortality.28,29 One could therefore expect to find a decrease in the rates of death and myocardial infarction in the long-term follow-up of drug-eluting stents due to a reduction in repeat revascularisation. However, our analysis and data from large-scale real world registries shows that there is no benefit at four years on these hard clinical endpoints. It remains to be determined whether the long-term restenosis reduction gained with DES use, outweighs a possibly slightly higher incidence of late stent thrombosis5,30-32.
Recent concerns were raised about the long-term safety of sirolimus-eluting stents in patients with diabetes mellitus. In a pooled analysis of the pivotal randomised sirolimus-eluting stent trials, a significantly reduced survival rate was found among diabetic (but not nondiabetic) patients treated with sirolimus-eluting stents17. Analysis of the causes of death and the occurrence of stent thrombosis in this high-risk subset could not adequately explain the observed difference in survival, and the four-year survival rate of diabetic patients receiving bare-metal stents was surprisingly high as compared to previous studies or the present analysis (95.6% versus 90.1% respectively). Nevertheless, in the present analyses, the overall survival was similar between the bare metal and paclitaxel-eluting stent group and although larger trials with long-term follow-up in diabetics are needed to settle this issue, there seems so far no cause of concern for a reduced safety of drug-eluting stents in diabetics.
The present analysis exclusively studied the slow-release PES, which is the only commercially available TAXUS stent. In the TAXUS II trial, the moderate-release PES, characterised by an three-fold greater amount of in vivo drug release over the first 30 days, proved to have a similar efficacy as the slow-release PES and the authors thereby suggested that the dosing threshold for prevention of restenosis had already been reached with the slow release version, at least for low-risk lesions8.
Several limitations need to be addressed. The present analysis was underpowered to detect a clinically significant difference in mortality, however, it is worth mentioning that ten thousand patients would be needed based on the results of the present study. Treatment with clopidogrel was required for at least six months by the original trial protocols, but no information on actual individual patient use was available, especially in patients with adverse events. Thus, we cannot provide any specific insight into the question of whether further prolonging dual antiplatelet therapy would reduce the risk of such events. We performed multiple subgroup analyses, including those for diabetes and complex lesions, which were not pre-specified. The number of fatal events in these subgroups was numerically small, so that the findings may still be due to the play of chance.
In summary, in this pooled analysis of four randomised trials, we compared the effects of paclitaxel-eluting stents with those of bare metal stents on clinical events at four years. No significant differences in the rates of death, myocardial infarction or stent thrombosis were demonstrated.
Acknowledgements
The authors would like to thank Drs. D. Baim, J. Koglin and P. Lam (Boston Scientific, Natick, Mass, USA) for their excellent assistance in the transfer of the data and Drs. E. Grube, A. Colombo, S. Ellis and G. Stone for their historical roles as the principal investigator of the four randomised trials: TAXUS I, TAXUS II, TAXUS IV and TAXUS V.
The authors had unrestricted access to the data and made all decisions about publication independently of the sponsor and the principal investigators. Dr Serruys assumes overall responsibility for the integrity of the data, the accuracy of the data analyses, and the completeness of the material reported.
The contribution of the authors is as follows. Drs J Daemen and P Serruys contacted the sponsor of these trials (Boston Scientific, Natick, Mass, USA) and the patient level data was transferred and subsequently analysed by two independent statistical institutions (Drs Olivier Varenne and Sophie Jacob, Paris Medical School, Paris, France and E Boersma, Erasmus University, Rotterdam, The Netherlands). The results were analysed by J Daemen, C Spaulding and P Serruys. The manuscript was written by Drs J Daemen, C Spaulding and P Serruys. All authors agree that two of them (C Spaulding and J Daemen) have contributed equally to the work. The authors had full access to the data and took decisions regarding analysis and publication independently of the sponsor.
The disclosures of interest are as follows: Dr Spaulding reports having received consulting or lecture fees from Cordis, Johnson & Johnson and Dr Varenne reports having received consulting or lecture fees from Cordis, Johnson & Johnson, Abbott and Boston Scientific. The studies were sponsored by Boston Scientific. Drs. Daemen, Boersma, Jacob, and Serruys have no disclosures.

