Abstract
Aims: To evaluate a new generation, thin strut, stainless steel Tsunami™ coronary stent in a contemporary percutaneous coronary intervention (PCI) practice across a wide geographical area.
Methods and results: Patients (n=1,437) with single or multiple vessel coronary artery disease undergoing PCI in 82 sites in Europe, Asia, and Australia were enrolled in the MATSURI registry. Clinical follow-ups were scheduled at 1, 6 and 18 months. Primary endpoint was major adverse cardiac events (MACE) rate at 6 months.
Diabetes was present in 25% of patients, 40% had unstable angina, 54% multivessel disease and 31% previously underwent PCI/CABG. Procedural success was achieved in 98.1% of patients. MACE rate was 1.8%, 7.3%, and 12.6% for 1, 6 and 18 month follow-up, respectively. At 6 months, the incidence of cardiac death, MI and TLR were 1%, 1.9% and 4.5% respectively, and confirmed stent thrombosis occurred in 0.2% of patients. Lower risk patients (43% of registry population) had six months MACE free survival of 96.7%.
Conclusions: Based on the results of this large registry we can conclude that the Tsunami™ BMS combines excellent deliverability and safety for all, together with very low MACE rates for lower risk patients. Long term follow-up confirmed sustained clinical benefit.
Introduction
Over the last decade coronary stents have revolutionised the field of interventional cardiology and have become the default treatment for percutaneous coronary intervention. However, the long-term success of coronary stenting is hampered by exaggerated neointimal formation resulting in in-stent restenosis. A large body of evidence has demonstrated that stents coated with antiproliferative drugs are the most effective way to address this problem. The efficacy of drug eluting stents (DES) has been established in comparative studies versus the older generation of bare metal stents (BMS) and striking differences, mainly related to reduction of repeat revascularisation, have completely overshadowed the latest developments in bare metal stent technology1-4. General use of drug eluting stents, however, remains limited by their high cost and need for extended dual antiplatelet therapy. Moreover, the antiproliferative properties of currently available DESs prevent or delay vessel healing which could be responsible for the late stent thrombosis frequently associated with increased incidence of myocardial infarction and death5-10. Healthcare funding agencies and interventional cardiologists face the challenge of properly selecting patients for this promising treatment.
Therefore, it is essential to generate further scientific evidence to identify patients/lesions with increased risk for in-stent restenosis and contribute to adequate selection of the treatment modalities for each individual patient. Likewise, the search for a better BMS design which can be used as a platform for DES development is of fundamental importance for further refinement of this breakthrough technology.
The primary aim of this large, prospective, multicentre, multi-continent, registry was to evaluate the performance of newly developed, thin strut, Tsunami™ (Terumo Corp. Tokyo, Japan) coronary stent system in daily clinical practice across a wide geographical area.
Methods
Patient population
Patients who were at least 18 year of age, had stable, or unstable angina or silent ischaemia, were considered for enrolment. Patients were excluded if they had left main disease, left ventricular ejection fraction of less than 30%, bifurcation requiring stenting of a diseased side branch, acute myocardial infarction in the preceding 48 hours, or intolerance to stainless steel, aspirin, clopidogrel, ticlopidine, heparin, or contrast media. The investigators were advised to use Tsunami™ for all consecutive patients complying with inclusion criteria.
Written informed consent was obtained from all patients prior to their inclusion into the study.
Lesions were treated with the use of standard interventional technique. Dual antiplatelet therapy with clopidogrel (75 mg once daily), or ticlopidine (250 mg twice a day), supplemented with 80-150 mg aspirin was recommended for four weeks. Aspirin alone was recommended for at least six months.
The Tsunami™ stent system
The Tsunami™ stent system is a stainless steel tubular stent with a double linked diamond cell structure, pre-mounted on a semi-compliant, low profile balloon. Low strut thickness of only 80 µm and short overhang of the balloon over the stent edges are designed to reduce vessel wall injury.11,12
The Tsunami™ coronary stent system was available in 2.0, 2.25, 2.5, 3.0, 3.5 and 4.0 mm diameters and 10, 15, 20, 25 and 30 mm lengths.
Endpoints of the registry
The primary endpoint of this registry was a composite of major adverse cardiac events (MACE) at six months after the procedure. Secondary endpoints were angiographic and procedural success rates, MACE rate at 18 months, target lesion revascularisation (TLR) at six months and in-stent thrombosis at 30 days.
Definitions
MACE was defined as composite of cardiac death, myocardial infarction (MI), coronary artery bypass grafting (CABG) or percutaneous target lesion revascularisation (TLR). Deaths were classified as cardiac or noncardiac. Deaths from undetermined causes were reported as cardiac. MI was defined either as the development of pathological Q-waves in at least two contiguous leads with or without elevated cardiac enzymes or, in the absence of pathological Q-waves, as an elevation in creatinine kinase levels to greater than twice the upper limit of normal in the presence of an elevated level of CK-MB fraction. Angiographic success was defined as final residual stenosis of the target site < 50% using the assigned treatment device alone. Procedural success was defined as angiographic success without in-hospital major adverse cardiac events.
Stent thrombosis was defined as angiographically confirmed thrombus within the stented vessel or any death (without other obvious cause), or Q-wave myocardial infarction in the territory of the stented segment within the first 30 days. It was classified as acute if it occurred within the first 24 hours, subacute up to 30 days, and late after 30 days. All reported re-interventions inside the stent and 5 mm proximally and distally were classified as TLRs. Other repeated PCIs in the same vessel were recorded as non-TLR target vessel revascularisations (TVR). An independent clinical event committee (see appendix) adjudicated all events.
Quantitative coronary angiograms obtained before and after the procedure were analysed at the independent core laboratory (Corisis, Paris, France) using a validated edge detection system (Medis-H, CMS 4.0, Leiden, The Netherland). Baseline angiographic variables included reference vessel diameter (RVD), minimal luminal diameter (MLD), percent diameter stenosis, lesion length and lesion complexity as defined by the American Heart Association/American College of Cardiology (AHA/ACC) classification. In-stent diameter stenosis, RVD, MLD, acute gain (final MLD-pre MLD) and the presence of dissection were noted post-procedure. No systematic angiographic follow-up was scheduled for this study. However, CD-ROMS for all performed repeat angiographic procedures were provided by the sites, analysed by the same core laboratory and adjudicated by clinical event committee.
Follow-up
All patients were scheduled to have one- and six-month clinical follow-up. 656 patients enrolled in 34 hospitals were subsequently re-consented for long term follow-up which took place at 18±5 months. At the time of follow-up contact, data were collected pertaining to current clinical status, concomitant drug therapy, and interim occurrence of adverse events.
Statistical and analytical plan
Statistical analysis was done by DatCom (Bekkevoort, Belgium) using SAS for Windows version 8.02 (SAS Institute, Cary, North Carolina, USA). All the analyses were based on the intention to treat population.
This was an observational, non-randomised study; therefore, the statistical analysis was based on descriptive statistical techniques. Categorical variables were presented as a rate with its 95% exact confidence interval, whenever appropriate. Continuous variables were presented as means ±1 standard deviation with their 95% confidence interval.
Single- and multiple-variable logistic regression analyses were used to derive predictors of MACEs. Multiple-variable predictors were chosen by a stepwise procedure. Probability values < 0.05 were considered to indicate statistical significance.
Results
Patient’s demographics
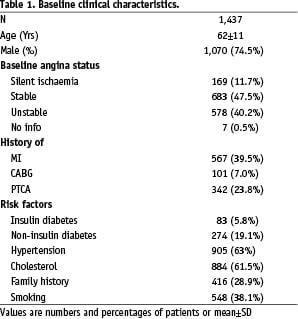
Eighty-two centres located across 27 countries in Europe, Asia, Africa and Australia enrolled 1,437 patients between January and September 2003. Among these patients, 883 (61.4%) were treated in Europe, 30 (2.1%) in Africa, 181 (12.6%) in Australia, and 343 (23.9 %) in Asia. Patient’s demographics are given in Table 1 and they are representative of a contemporary PCI practice.
Lesion characteristics
QCA data were available for 1,496 of 1,792 lesions (83%). Unavailable or unreadable CDs account for missing values.
Subsequent analysis showed no difference in baseline demographics and MACE rate between patients with and without angiographic evidence.
Main lesion characteristics are presented in Tables 2 and 3.
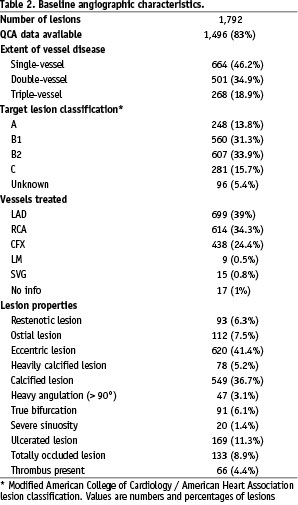
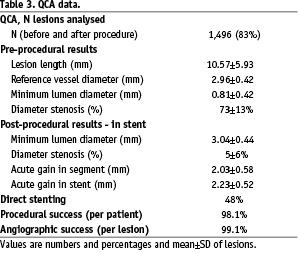
One half of the lesions were categorised as B2 and C type. Multivessel coronary artery disease was recorded in 54% of patients. The reference vessel diameter was less than 2.75 mm in 33% of treated lesions with available QCA data.
Procedural characteristics
Predilatation was performed in 52% of the lesions while postdilatation was needed for 24% of the lesions. A total of 1,792 lesions were treated with 1,896 stents, representing 1.25 lesions and 1.31 stents per patient. Stent length ranged between 10 and 30 mm and their diameters between 2 and 4 mm. A single lesion was treated in 1,082 (75%) patients, and multiple lesions in 355 (25%) patients. In 5.6% of patients other bare metal stents, drug eluting stents, or balloon angioplasty were used alone or in combination with Tsunami™ stent for the treatment of multiple lesions.
Residual diameter stenosis of less than 50% was achieved in 99.1% of the lesions and less than 30% in 95% of the lesions. Overall rate of in-hospital major adverse cardiac events was 1.3%. There was one acute in-stent thrombosis (0.07%) during the procedure.
Clinical follow-up
One month follow-up compliance rate was 96.4% (1,385 patients) and 827 (57.5%) patients were on dual antiplatelet therapy. MACE rate between index hospitalisation and 30 days was 0.5% (Table 4).
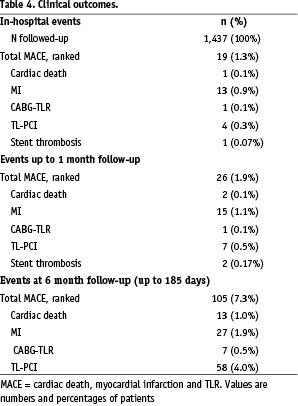
Two subacute stent thromboses were recorded.
Between one and six months, 79 patients experienced MACE (5.5%), the majority represented by target lesion revascularisation (4.0%). Out of 105 patients experiencing MACE up to 6 months, 15 (14.3%), were treated with stents other than Tsunami™, 15 (14.3%) patients had multiple stents implanted in one lesion and 14 (13.3%) had treated complex true bifurcation or unprotected left main artery. Diabetic patients comprised 38% of patients with MACE. There were no cases of confirmed late stent thrombosis. TVR rate was 4.5%.
MACE rate for patients with diabetes mellitus (355 patients) was 11.3% and was significantly (p=0.013) higher than for non-diabetic patients (6%). Nine of 13 deaths in this registry were amongst diabetic patients and these patients were significantly older and comprised a significantly higher proportion of females.
Female gender was associated with a trend towards higher MACE rate but the difference did not reach statistical significance (8.9% for female, versus 6.9% for male).
MACE rate of 8.3% in 432 patients with 480 target lesions in small vessels (RVD<2.75 mm) was not significantly different from 7.1% MACE rate observed in the patients with larger vessels.
Patients with treated multiple lesions had MACE rate 9.8% which was significantly higher (p=0.012) than 6.5% MACE rate in patients with single lesions.
Long term follow-up
Out of 656 patients (716 lesions) that gave informed consent to attend long term follow-up, 633 (96.5%) patients were available for clinical assessment (Table 5).
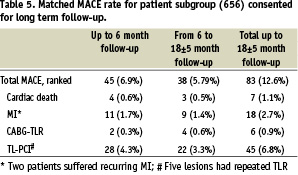
Clinical and angiographic characteristics of patients assigned for long term follow-up were similar to overall registry population. MACE rate for this group of patients increased from 6.9% at 6 months to 12.6% at 18±5 months. There were 3 new cardiac deaths, 9 MIs in 7 patients, and 26 target lesion revascularisations in 21 patients. There were no cases of late stent thrombosis.
Risk factor analysis
A total of 15 demographic, clinical, angiographic and procedural variables were entered into the regression-analysis model in search for predictors of adverse clinical events (Table 6).
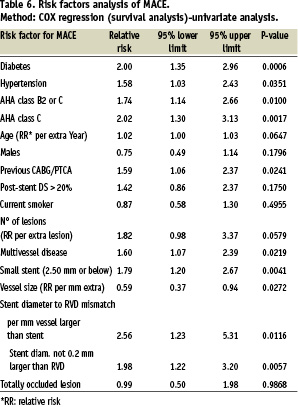
By multiple variable analysis, 4 characteristics emerged as highly predictive for overall MACE and the fifth (hypertension) was on the limit of significance (Table 7).
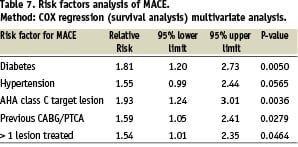
A categorical assignment of all the patients into 3 subgroups relative to the number of risk factors, revealed that 612 (42.6%) patients with one or none of the risk factors enjoyed a MACE free survival of 97.3% at 6 months, contrary to 78.7% in 90 (6.3%) patients with 3 or more risk factors (Figure 1).
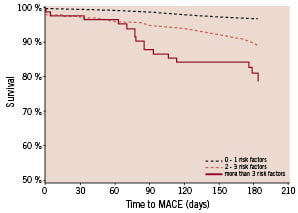
Figure 1. Subgroups: Group 1: 0 or 1 risk factor; Group 2: 2 or 3 risk factors; Group 3: more than 3 risk factors.Survival at 6 months: Group 1: 96.7%, 95% CI [95.2% - 98.2%] (N=612); Group 2: 89.0%, 95% CI [86.6% - 91.4%] (N=735); Group 3: 78.7%, 95% CI [69.2% - 88.3%] (N=90)
Discussion
The main findings of our study are excellent deliverability (99.1% angiographic success rate) and clinical safety and efficacy of Tsunami™ stent in short and long term follow-up. Stratification of the patients according to the number of risk factors, identified by multivariate analysis, showed that by appropriate patient selection a very low MACE rate could be achieved with this type of new generation bare metal stent.
Long term MACE free survival was also high and, as expected, a decline in the TLR rate from 6 to 18 months was observed, without increase in the rates of death and MI13-15. This finding is in agreement with the hypothesised temporal sequence of restenosis which peaks at 3 to 4 months after BMS implantation and may regress thereafter. The timing of the healing process appears to be different from that observed after DES implantation where it could be responsible for late stent thrombosis9. However, despite the decrease over time some patients in our study still experience a need for revascularisation of target segment probably inherent to the progression of the disease.
Stenting of small vessels yielded results which appear superior to other BMSs and similar to those observed by Ardissino et al (9.3% MACE rate) for the Cypher arm in the SES-SMART study and Danzi et al for Tsunami™ stent in small vessel registry11,16-20. It can be speculated that the particularly thin stent struts (80 µm) of the Tsunami™ coronary stent, and its design, contributed to the good clinical performance. The patients enrolled in this registry did not have angiographic follow-up which could have resulted in reduced TLR. The phenomenon of increased TLR rate with protocol mandated angiographic follow-up is both well known, and well described in several studies21. However, low late loss, of only 0.58 mm, reported for Tsunami™ stent by Bonnier et al is comparable to reported late loss in some DES studies indicating that Tsunami™ stent may induce lower vessel wall injury and neointimal proliferation12.
Our results compare favourably with the new generation bare metal stents. The Trimaxx ™ stent showed a 10% MACE rate in a registry involving very simple lesions in larger vessels (RVD 3.0-3.75 mm), while a MACE rate of 14.7%, and a stent thrombosis rate of 1.2% were reported for the Driver™ stent in the ENDEAVOR II randomised trial, which enrolled patients similar to those of our MATSURI registry22,23.
Long term clinical outcomes after placement of the Tsunami™ stent are particularly appealing in the light of the recently reported results of the Late Basket trial, where the incidence of death and MI in the patients treated with DES increased over time and exceeded the corresponding incidence in the BMS arm24.
The low incidence of cardiac death, myocardial infarction, and stent thrombosis, recorded in our registry indicate that a highly deliverable, thin strut, modern generation bare metal stents could still represent a valuable option for the treatment of well selected patients with lower risk of restenosis. As such, exposure of those patients to a long term dual antiplatelet therapy, and the possible threat of late stent thrombosis, could be avoided without compromising clinical outcomes.
Study limitations
Lack of randomisation could be considered as one of the limitations of this study. However, despite the reliability of randomised trial results they frequently exclude patients at the highest risk for complications and those who are least likely to show benefit, reducing as such the resemblance to real clinical practice. Absence of angiographic follow-up could represent another limitation of this study however, the value of clinical endpoints which are best assessed through registries, such as MATSURI, may be of greater relevance for assessing patient outcome. Similar to the other large multicenter registries, the potential under reporting of adverse events due to the lack of routine monitoring could be considered as one of major limitation of our registry. However, previously conducted, well controlled, clinical trial with Tsunami™ stent reported an incidence of death and MI of only 2% at 6 month follow-up, suggesting that under reporting of adverse events was not a major limitation of this registry21. Although advised to do so, not all investigators enrolled consecutive patients and as such biased patients selection can not be ruled out.
Conclusions
The relatively low MACE rate observed in this registry indicates that modern generation thin struts bare metal stents are a safe and efficient treatment option for appropriately selected patients. A large number of participating centres and wide geographic representation assure that those results are truly representative of the real world, instead of patients selected along strict criteria in only the most experienced centres.
Acknowledgements
This study was supported by Terumo Europe N.V.
Appendix
Sponsor: Terumo Europe N.V.
Principal Investigator: Didier Blanchard, St. Gatien Clinic, Tours, France
Steering Committee: Didier Blancard, St. Gatien Clinic, Tours, France, Gian Battista Danzi, Poliambulanza , Brescia, Italy (currently Ospedale Maggiore, Milan, Italy), Philip Urban, Hopital de La Tour, Geneva, Switzerland, Eulogio Garcia, Hospital Gregorio Maranon, Madrid, Spain, Magali Breheret, Terumo Europe, Hirofumi Nagai Terumo, Corporation, Dragica Paunovic, Terumo Europe
Clinical Event Committee: Victor, Legrand, Centre Hospitalier Universitaire Sart Tilman, Liege, Belgium, Thierry Lefevre, Institut Cardiovasculaire Paris Sud, Massy, France, Nicolas Danchin, Hopital Europeen Georges Pompidou, Paris, France.
The following investigators participated in the MATSURI study:
EUROPE: Belgium: B. Peperstraete, MD, Brussels, M. Vincent, MD, Brussels; France: Y. Biron, MD, Rennes, D. Blanchard, MD (PI), Tours, T. Joseph, MD, Boulogne, H. Escojido, MD, Marseille, G. Finet, MD, Lyon, B. Glatt, MD, Saint-Denis, M. Hamon, MD, Caen, B. Huret, MD, Caen, J. Marco, MD, Toulouse, C. Picot Bourdonnec, MD, Rennes, V. Stratiev, MD, Paris; Germany: W. Auch-Schwelk, MD, Heppenheim, B. Hennen, MD, Homburg, H. Sievert, MD, Frankfurt, N. Reifart, MD, Bad Soden, K. Hauptmann, MD, Trier, T. Schiele, MD, Muenchen, B. Nowak, MD, Frankfurt/Main, A. Klein, MD, Ravensburg, Steindl, MD, Göppingen; Greece: D. Pentousis, MD, Thessaloniki, G. Triantis, MD, Athens, I. Zarifis, MD, L. Mosialos, MD, Thessaloniki; Israel: M. Mosseri, MD, Jerusalem, R. Kornowski, MD, Petah-Tikva; Italy: G.B. Danzi, MD, Brescia; G. Piva, MD, Ancona; M.Galli, MD, Como; C. Cernigliaro, MD, Novara; F. Della Rovere, MD, Genova; R. Bonmassari, MD, Trento; The Netherlands: H. Bonnier, MD, P. den Heijer, MD, Breda, M.J. Suttorp, MD, Nieuwegein; Denmark: L. Thuesen, MD, Aarhus, H. Kelbaeck, MD, Copenhagen, P. Thayssen, MD, Odense; Russia: V. Chestuchin, MD, Moscow, E. Tchebotar, MD, Nizhny Novgorod, H. Samko, MD, Moscow, V. Klimov, MD, Moscow, A. Serenko, MD, Khanty-Mansiysk, A. Protopopov, Krasnoyarsk; Spain: M. Recio, MD, Madrid, J. Moreu, MD, Toledo; Switzerland: P. Urban, MD, Geneva; United Kingdom: M. Thomas, MD, London
AFRICA: Morocco: S. Ztot, MD, Rabat
ASIA: Bangladesh: S. Rahman, MD, Dhaka; China: J.B. Ge, MDShanghai, D. Ho, Hong Kong; India: K. Punamiya, MD, Mumbai, A. Bhat, MD, Trivandrum, L. Krishna, MD, Hyderabad, B. Muralidhara, MD, Bangalore, S. Iyengar, MD, Bangalore; Indonesia: T. Santoso, MD, Jakarta, O. Rachman/M. Yusak Jakarta; Korea: SJ Park, MD, Seoul, BK Lee, MD, Seoul, JH Joon, MD, Seoul, MH Jeong, MD, Gwan Ju; Malaysia: K. H. Sim, MD, Sarawak, O. Ismail, MD, Penang, C. Y. Lee, MD, Johor; Philippines: E. Sison, MD, Manila; Singapore: Y. Lim, MD, Singapore; Sri Lanka: M. Rajakaruna, MD, Colombo; Thailand: D. Tresukosol, MD, Bangkok, W. Udayachalerm, MD, Bangkok, S. Tansuphaswadikul, MD, Bangkok
AUSTRALIA: G. Wilkins, MD, Dunedin, G. Cope, MD, Perth, J. Gutman, MD, Melbourne, R. Yeend, MD, Adelaide, A. Farshid, MD, Canberra, C. Juergens, MD, Sydney, D. Eccleston, MD, Melbourne

