Abstract
Aims: Failure to cross a calcified tight stenosis or chronic total occlusion (CTO) with a balloon after having passed a guidewire is a rare but serious intricacy with a high likelihood of failure. We report our experience of a three years period (December 2004-November 2007) using a novel metal penetration catheter (Tornus) to bail-out 44 failures to cross a lesion with a balloon catheter.
Methods and results: The Tornus (Asahi Intecc, Aichi, Japan), an over-the-wire metal exchange catheter that might be “screwed” counter-clockwise into a lesion, was used in 44/2881 PCI-patients with calcified and tortuous coronary anatomy in whom a 1.5 mm lubricious balloon catheter could not be pushed into the lesion despite optimal support of a 6 Fr guiding catheter and additional anchoring with a stiff-shafted guidewire (anchor wire) when applicable.
In 39/421 patients the target was a chronic total occlusion and in 5/2460 patients it was a stenotic lesion or recent occlusion. In these 44 consecutive balloon-failures the Tornus completely passed 35 lesions (79.5%) – all but one were successfully followed with a balloon and stent. In one patient, the balloon did not follow despite successful passage of the Tornus. In 5/9 patients in whom the Tornus passed incompletely, the channel was opened sufficiently to allow for successful passage with a balloon. In three failures to dilate after Tornus, the procedure was successfully finished with the use of a Rotablator. Three of the 44 finally failed. Over all, the Tornus procedure relevantly contributed to success in 91% of the cases with failure of balloon crossing. We experienced no complications or fractures.
Conclusion: The Tornus is an easy to use over-the-wire device to increase a coronary channel and enhance balloon entry of complex tight lesions and chronic total occlusions.
Introduction
The results of PCI in chronic coronary occlusions (defined as occlusion-age > 3 months) have improved dramatically in the last 15 years due to operator experience and improvement of material1.
The main reasons existing today for reduced success are limited operator experience, missing stump, hard fibrotic tissue or the presence of calcium, that increases with advanced lesion age2. In addition to calcification, the length of the occlusion and marked tortuosity appears to be an independent predictor of procedural failure as well3.
Despite tremendous improvements, the success rate of interventional treatment of CTO remains still significantly lower than in non-occluded lesions, the reasons for failure being manifold. In Kinoshita’s series of 397 patients with an estimated occlusion age of 2 to 170 months the success rate was 81%. Reasons for procedural failure were: inability to cross the lesion with a guidewire (63%), long intimal dissection with creation of a false lumen and residual stenosis >60% (24%), extravasation (11%), failure to cross the lesion with the balloon or dilate sufficiently (2%) and thrombus (1.2%)4.
We evaluated the most common reasons why the first PCI of CTO was not successful in 200 consecutive patients referred for a second attempt in 1998-2002. Similar to Kinoshita’s experience in most failed cases, the wire did not cross the lesion. In 9% of the cases, however, the balloon did not cross the occlusion because of poor support of guiding catheter and the tightness of the occlusion itself. In these 200 patients, the success rate of our second attempt was 71%, mainly due to different wires and wiring technology, better guiding support (e.g. second stiff-shafted anchor wire), various support techniques for stenting – and in 4% – the use of a Rotablator to modify the calcific ring and create space for the balloon5,6.
Several techniques have been described to enhance the support of a guiding catheter like the use of a 5 Fr system inserted in a 6 Fr guide7; exchanging the guiding catheter against larger and bigger ones for better support (with the risk of loosing wire position); slide a second stiff-tipped wire (e.g. Miracle 6g) beside the first, to enlarge the channel (plaque-crush). Instead of a stiff-shafted second wire, a second anchoring balloon might be inserted as well and inflated over a buddy wire in the proximal vessel, or a branch proximal to the occlusion, but this often requires al larger than 6 Fr guide, which is unpopular in Europe.
Two additional options are very effective but cumbersome due to wire exchange or very expensive: Rotablator8 and laser9. Since we abandoned the laser because of unacceptable costs for maintenance, our bail-out device for balloon failures until 2005 was rotablation. Since December 2004 we were able to additionally use the Tornus as a bail-out device.
Device description
The Tornus® (Asahi Intecc Corp. Japan), is a novel, over-the-wire very flexible tapered metallic exchange catheter designed for complex and tight coronary lesions10. The catheter can be steered manually through a very tight stenosis or chronic occlusion10. (Figure 1)
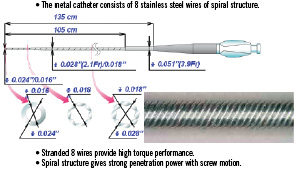
Figure 1. Structural feature of the Tornus.
The Tornus is available in 2.6 Fr and more recently in a 2.1 Fr more flexible version.
This metal catheter’s main shaft is a coreless stainless steel coil that consists of eight stranded stainless wires to cross through a severe stenosis by manual counter-clockwise rotation. The rotating energy will be stored in the catheter and eventually transformed into tip rotation and cork-screw like advancement through the lesion. Unlike the Rotablator, it is not supposed to ablate, but rather to penetrate and dilate to result in a slightly larger channel. It is recommended to release the torque energy after 20 rotations in order not to risk wire fractures that may cause severe problems in retrieving the device. After penetration and passage of the lesion, the Tornus may serve as an exchange catheter. To retrieve the Tornus, the catheter has to be turned clockwise. The inserted wire may be left in distal position with the assistance of a docking wire.
Patients and lesion morphology
During December 2004 – November 2007 we used the Tornus as a bail-out device in 44/2881 PCI-patients with calcified and tortuous coronary anatomy in whom a 1.5 mm lubricious balloon catheter could not be pushed into the lesion. In 39/421 patients the target was a chronic total occlusion aged > 3 months, TIMI flow 0, in whom the 1.5 mm Maverick balloon (Boston Scientific, USA) could not be passed over the wire that we were able to successfully navigate through the lesion. In five patients, it was a stenotic lesion or recent occlusion.
Calcification was classified angiographically as none (0); mild (1) i.e. spots of calcium; moderate (2) i.e. plaques of calcium larger than 3x vessel diameter, but not involving the circumference totally in two perpendicular views; and severe (3) i.e. > 3x vessel diameter and comprising the vessel wall totally in two perpendicular views. In 71% percent of the patients, the lesion calcification was moderate or severe, mean grade 1,9±0.87 (a scale of 0-3). Tortuosity grade 0-3 was defined as none (0) i.e. angulations <45; mild (1), i.e. at least two segments of the coronary arteries with curvatures > 45°; moderate (2), i.e. one segment > 90° or at least two segments of the coronary arteries with curvatures > 60°; or severe (3), i.e. two or more segments of the coronary arteries with curvatures > 90° measured during diastole.
The mean grade of tortuosity was 1.88±0.77, and the mean length of occlusion/stenosis was 37.6±18.7 mm.
In all patients, a well axially positioned 6 Fr guiding catheter was used via the femoral route. Prior to use of Tornus, 30/44 patients presenting with appropriate vessel anatomy had had placed a second guidewire (Ironman® Abbott Vascular, IL, USA) for better backup support, but still the balloon would not cross, while in 14 patients the vessel proximal to the occlusion did not permit an anchoring technique. In none of the patients did we attempt to exchange the guiding catheter (e.g., to a larger size) in order not to risk losing the wire position, nor did we try to dilate the lesion partially as far as the balloon could be pushed. This latter technique sometimes allows the operator to then take advantage of a larger lesion entry with a new balloon.
The Tornus was used in 32 right coronary arteries, seven circumflex arteries, three left anterior descending arteries and two diagonal branches.
Results
In 35 patients, the 2.6 Fr device was the only one used, in nine patients a 2.1 Fr device was applied and in five of these latter patients the 2.1 Fr device had to be followed by a 2.6 Fr device to further increase the lumen. The Tornus could easily be advanced to all lesion entry points and could be retrieved in all cases without device fracture (See study schematic).
Of these 44 consecutive patients, the Tornus passed the lesion completely in 35 (79.5%) – all but three were successfully followed with a balloon and all but four with one or more stents. In one patient, the balloon did not follow despite successful passage of the Tornus and a Rotablator was used successfully. In one patient, three 1.5 Maverick balloons entered the calcified CX-lesion but ruptured at pressures between 4 and 6 atm. A 2 mm, high pressure balloon could only pass the lesion after preparing the channel with a 2.6 Fr Tornus and then we were further able to expand it at 24 atm.
In nine patients the Tornus failed to cross, and passed only incompletely but opened the channel enough in five of these patients to allow successful passage with a balloon. In three failures, the procedure was successfully finished with the use of a Rotablator. Three of 44 cases remained unsuccessful, because the Rotablator-wire could not be placed, or a Rotablator appeared contra-indicated because of severe tortuosity, or because a stent could not be placed. Over all, the Tornus relevantly contributed to success in 91% of the cases with failure of balloon crossing.
Failure to completely pass the lesion with Tornus
1. CTO of RCA, occlusion age 144 months, occlusion length 52 mm, moderate calcification mild tortuosity,
2. CTO of CX, occlusion age undefined, occlusion length 14 mm, severe calcification, severe tortuosity
3. CTO of RCA, occlusion age undefined, occlusion length 48 mm, moderate calcification, moderate tortuosity
4. CTO of RCA, occlusion age 230 months, occlusion length 56 mm, severe calcification, severe tortuosity (a finally unsuccessful case)
5. CTO of CX, occlusion age undefined, occlusion length 15 mm, severe calcification, severe tortuosity
6. CTO of D1, occlusion age 136 months, occlusion length 25 mm, severe calcification, moderate tortuosity (a finally unsuccessful case)
7. CTO of RCA, occlusion age undefined, occlusion length 44 mm, severe calcification, moderate tortuosity
8. CTO of RCA, occlusion age 32 months, occlusion length 39 mm, severe calcification, mild tortuosity
9. CTO of RCA, occlusion age 228 months, occlusion length 82 mm, moderate calcification, severe tortuosity (a finally unsuccessful case)
Failure to pass the Tornus completely through a lesion in these nine patients was most likely due to the severe calcification and tortuosity of the long chronic occlusion.
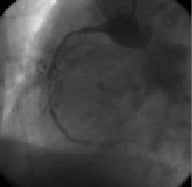
Figure 2. RCA – Occlusion with bridging collaterals, mild calcification, no tortuosity 6 Fr AL1 guiding catheter.
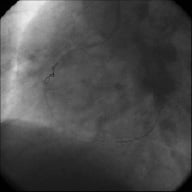
Figure 3. Wire past occlusion and second wire (Ironman) proximal to occlusion to support guiding catheter.
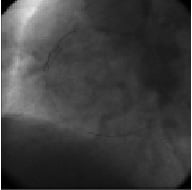
Figure 4. The Guiding catheter backs while trying to pass the 1.5 Maverick balloon.
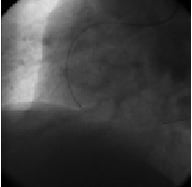
Figure 5. Tornus advanced through occlusion.
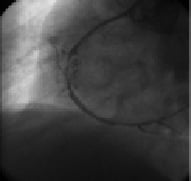
Figure 6. Channel achieved by Tornus.
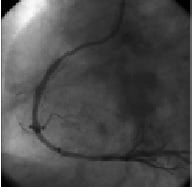
Figure 7. Final results after stenting.
Discussion
The requirement of Tornus in 9% of our patients with CTO may be flawed by the ease to use the device. A more vigorous attempt to overcome the resistance against a 1.5 mm Maverick e.g., using multiple small balloons sequentially or sliding a stiff wire parallel to the first might eventually have been successful as well. Likewise superior balloons (e.g. Ryujin 1.25 mm) are now available also, and will likely reduce the need for Tornus in our hands. Interventional centres who still have access to the Excimer Laser system might successfully use a small over the wire laser catheter to enlarge the channel. Because of the tremendous costs to maintain such a system we decided to abandon the technique in 2000.
The majority of our cases described here were patients with occlusion of the RCA, which reflects the anatomical problem of getting sufficient guiding support namely with proximal occlusions. In these patients larger guides (7 Fr or 8 Fr) or stronger guides (left Amplatz) are considered from the beginning, this strategy however may lead to an occlusive proximal dissection or impede wire manipulation because of guide-misalignment in the case of very proximal occlusions or ostial disease. We therefore often prefer a less traumatic guide (e.g. 6 Fr Amplatz R2 or IMA) with the downside of yielding less passive support. An alternative method to increase the backup of a guiding catheter is to insert a 5 Fr longer soft-tipped catheter into the 6 Fr guiding7. This is, however, also not applicable in very proximal occlusions.
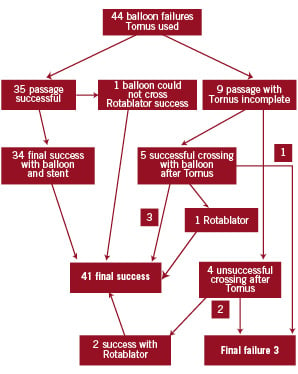
Figure 8. Study schematic.
Conclusion
In our recent cohort of patients with complex coronary artery occlusions the Tornus had to be applied in 9% of the cases with old chronic occlusion and 0.1% of non-CTO-lesions because of balloon failure. The Tornus device was easy to use, safe and and increased the success rate of these failures to 91%. The Tornus may fail to pass very tortuous and severely calcified lesions. Despite possible device fracture and failure to retrieve it, we experienced no complications related to the Tornus catheter.
In summary, while there are many ways to overcome the problem of failure to cross tight lesions or occlusions with a balloon catheter, according to our experience, the Tornus catheter is one of the most simple and straight forward strategies.

