Abstract
Severe calcification is frequent in coronary chronic total occlusions (CTO), and its presence has been associated with increased procedural complexity and poor long-term outcomes following percutaneous coronary intervention (PCI) in an already challenging anatomical setting. The diagnostic characterisation of heavily calcified CTOs using non-invasive and invasive imaging tools can lead to the application of different therapeutic options during CTO PCI, in order to achieve adequate lesion preparation and optimal stent implantation. In this expert review, the European Chronic Total Occlusion Club provides a contemporary, methodological approach, specifically addressing heavily calcified CTOs, suggesting an integration of evidence-based diagnostic methods to tailored, up-to-date percutaneous therapeutic options.
Introduction
Chronic total occlusions (CTO) remain one of the most complex scenarios in contemporary percutaneous coronary interventions (PCI), with implicit technical difficulties and the need for high-volume complex PCI centres and operators who continuously refine their skills. Unfavourable histopathological features of CTOs, such as the presence of severe calcification, increase the complexity of treatment. In particular, heavy calcification (HC) may preclude wire advancement within the intraplaque space of the occlusion, forcing the operator to use extraplaque techniques that are associated with higher periprocedural risk and a potentially poorer outcome12. In addition, even after a successful wire crossing, advancing balloons and deploying stents can become challenging. These intraprocedural difficulties can potentially have a detrimental impact on patient prognosis, since poor stent expansion is a major predictor of stent thrombosis and restenosis34.
The high prevalence of calcium in CTOs warrants the utilisation of specific strategies and techniques to ensure appropriate lesion preparation. Accordingly, the detection and quantification of HC in CTOs is a prerequisite for selecting the most efficient recanalisation technique. The preprocedural assessment of calcium burden and its location within the CTO can be obtained by using coronary computed tomography angiography (CCTA). After wire crossing, characterisation of the lesion anatomy with intravascular imaging can further offer invaluable insights to guide tool selection for a tailored lesion preparation5.
This paper provides a comprehensive overview of contemporary interventional management of heavily calcified CTOs, suggesting an integrated diagnostic/therapeutic approach to guide the choice between available treatment options and optimise procedural results.
Impact of coronary calcification on clinical outcomes
The presence of severe calcification in the setting of chronically occluded epicardial vessels negatively affects clinical outcomes. Stent delivery through heavily calcified, atherosclerotic coronary arteries can induce polymer damage and cause impaired drug delivery, paving the way to restenosis and stent thrombosis6. In a retrospective cohort of 285 patients undergoing CCTA before CTO PCI, Ito et al showed that CTO PCI in patients with severe calcification resulted in higher rates of in-stent restenosis and target lesion failure7. Severe calcification emerged also as an independent predictor of adverse outcome.
Karacsonyi et al assessed 1,453 consecutive CTO PCI patients and showed that moderate-to-severe coronary calcium was associated with lower technical and procedural success rates8. The incidence of major adverse cardiac events was significantly higher in the subset of patients with heavily calcified CTOs (3.7% vs 1.8%; p=0.033). Furthermore, the presence of heavily calcified CTOs was associated with longer procedural and fluoroscopic times, higher radiation dose and contrast medium volume and required the use of the retrograde approach more often. Procedural complications, mainly pericardial tamponade, were more frequently encountered because of the higher adoption of dissection techniques, dictated by the presence of severe calcification.
Histopathology of calcification in chronic total occlusions
The process of calcification is initiated by smooth muscle cell apoptosis and perpetuated by the apoptosis of infiltrating macrophages910. Srivatsa et al showed that the content of calcium increases with advancing age of the CTO11. Although intimal plaque calcification is the predominant type of calcification seen in coronary arteries, medial calcification has been observed in patient subgroups and was associated with renal failure, hypercalcaemia, hyperphosphataemia and parathyroid hormone abnormalities10.
Understanding the histopathological distribution of calcium may have an important impact on guiding percutaneous treatment of heavily calcified CTOs. Sakakura et al performed a histopathological comparison of 95 CTO lesions from 82 patients, dividing them according to the CTO duration and the presence of anastomoses of coronary artery bypass grafting (CABG)12. They showed that the calcific burden was heavy in patients with previous CABG, intermediate in long-standing CTOs without CABG, and mild to moderate in more recent CTOs. Extensive calcification was seen in post-CABG patients, in line with previous studies, suggesting a more rapid progression of atherosclerosis in grafted coronary segments13. The authors suggest that competitive flow, resulting in blood stasis and reduced shear stress, can be the underlying mechanism of more severe calcification present in post-CABG CTOs. These findings suggest that the lower success rate observed in post-CABG CTO recanalisation may be caused by more extensive calcification involving the index occlusion1415.
Overall, severe calcification is more common in native CTOs compared to in-stent (IS) CTOs. Mori et al identified 56 lesions in 54 different patients with IS CTOs and showed that neointimal calcification was observed in only 3 lesions with IS neoatherosclerotic rupture and 1 lesion in an IS calcified nodule, all of which occurred in bare metal stents10. Furthermore, only 4% of IS CTO lesions had 10% of neointimal calcification of the area/stent area, which is significantly lower compared to the 48% previously found in native CTO lesions by Sakakura et al12. However, given the fact that the second-generation drug-eluting stent era is rather recent, neoatherosclerosis with severe calcification may be found in future secondary occlusions of previously treated lesions.
CTO scores and crossing algorithms – the impact of calcium
Scoring systems have been developed to predict procedural success and efficiency in CTO PCI (Table 1). The Japan CTO (J-CTO) score, introduced in 2011 by Morino et al, remains the most widely used CTO score to date, mainly due to its simplicity and ease of use. This model is used to predict the probability of successful wire crossing within 30 minutes; calcification, detected angiographically within the CTO segment, has emerged as an independent predictor of complexity16. The Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS-CTO) score was developed to estimate technical success using the “hybrid approach”17 and was validated in a patient population treated by experienced operators in high-volume centres of excellence14. In this case, calcification did not emerge as a predictor of procedural failure, probably due to the liberal and early use of extraplaque dissection techniques that potentially “bypassed” intimal calcification. On the contrary, severe calcification emerges as an important component of the European CTO (Euro CTO) score, which was derived from the largest dataset currently available (14,882 patients).
Several algorithms have been introduced to establish a systematic approach in CTO crossing and to optimise the use of equipment and resources. The first algorithm was the hybrid algorithm by Brilakis et al, in which severe calcification was not considered for decision-making17. Other algorithms in use, such as the Euro CTO Club algorithm and the Asia-Pacific CTO Club algorithm, consider heavy calcification as a criterion to primarily use “knuckle wiring”, which involves the utilisation of the extraplaque space to achieve CTO crossing1819. As a result of worldwide consensus, the Global Chronic Total Occlusion Crossing Algorithm has been introduced recently, recommending retrograde dissection/re-entry over retrograde wiring in cases where severe calcification, amongst other criteria, is present20. The above-mentioned algorithms acknowledge the presence of HC within the CTO segment as an important obstacle to overcome and propose using dissection/re-entry techniques to facilitate CTO crossing.
Table 1. The role of calcium in different CTO scores.
| Name of score | J-CTO score | PROGRESS-CTO score | CL score | EuroCTO (CASTLE) score | KCCT score# | CT-RECTOR score# |
|---|---|---|---|---|---|---|
| Role of calcium | Any calcification within the CTO segment (1 point) | - | Severe calcification (2 points) | Severe calcification (>50% of the CTO segment) (1 point) | - Peripheral calcification* (1 point)- Central calcification** (2 points) | Calcification ≥50% CSA within CTO |
| #The definition of calcium in the KCCT and CT-RECTOR scores is performed using computed tomography; all other scores define calcium angiographically. *Maximal encircling ≥180 and CSA ≥50%. **CSA=100%. CASTLE: Coronary artery bypass graft history, Age, Stump anatomy, Tortuosity degree, Length of occlusion, and Extent of calcification; CL: clinical- and lesion-related; CSA: cross-sectional area; CT: computed tomography; CT-RECTOR: Computed Tomography Registry of Chronic Total Occlusion Revascularization; CTO: chronic total occlusion; EuroCTO: European CTO Club; J-CTO: Multicenter CTO registry in Japan; KCCT: Korean Multicenter CTO CT Registry; PROGRESS: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention | ||||||
The role of imaging in heavily calcified chronic total occlusions
Coronary computed tomography angiography
Use of CCTA in patients with CTO is limited by issues relating to patient radiation exposure and reimbursement costs. However, CCTA, particularly in cases of a heavily calcified CTO, can offer invaluable non-invasive diagnostic insights when used as a tool for preprocedural diagnostic assessment (Figure 1). Of note, CCTA is able to overcome most of the diagnostic limitations of dual coronary angiography, allowing a complete anatomical description of the coronary arteries involved, including the occluded segments21. Fujino et al assessed the diagnostic accuracy of a CCTA-derived J-CTO score versus the conventional angiographically derived J-CTO score22: the diagnostic accuracy of the CCTA-derived J-CTO score for predicting procedural success and 30 min wire crossing was significantly higher than the J-CTO score derived from conventional angiography. In order to overcome the problem of the high sensitivity of CCTA in detecting calcium, the authors used a cut-off of a calcified area >50% of the vessel cross-sectional area to indicate clinically meaningful calcification. In addition, new data presented by Hong et al show higher procedural success rates and fewer complications for patients randomised to CT-guided CTO PCI versus patients without CT guidance23. Notably, the impact was greater for patients with more complex CTO anatomies, expressed as higher J-CTO scores (≥2).
CCTA is able to identify the calcium distribution within the coronary vessel structure. Circumferential calcification, defined by calcification encircling 360° and occluding the whole vessel lumen (cross-sectional area CSA=100%; “full moon”), was independently associated with a wire-crossing time >30 minutes and procedural failure. Accordingly, it was made part of the Korean Multicenter CTO CT Registry (KCCT) score24. Along the same lines, the Computed Tomography Registry of Chronic Total Occlusion Revascularization (CT-RECTOR) study showed that a CT-identified calcification >50% of the CSA within the CTO segment is an independent predictor of failed wire crossing within 30 minutes25.
Preliminary results using real-time CCTA fusion showed the feasibility of a CT scan and fluoroscopy coregistration, highlighting the potential of CCTA to guide online CTO PCI. Accordingly, percutaneous revascularisation techniques can be adapted to the calcium distribution determined by CCTA. Ghoshhajra et al showed that antegrade dissection re-entry (ADR) can be effectively guided by coregistered CCTA, in order to identify a calcium-free vessel segment to achieve successful wire re-entry from the extraplaque space into the true vessel lumen26.
Despite the advantages associated with the use of CCTA in the context of heavily calcified CTOs, inherent limitations of the technique may reduce image quality. First, the lack of resolution, depending on the CT scanner used, can heavily affect the definition of critical characteristics of a heavily calcified CTO lesion such as the calcium thickness. Second, heavily calcified coronary arteries produce rendering and volume artefacts on CT imaging, known as a “blooming effect”27. This phenomenon results in an erroneous enlargement of the calcified segment and remains a major challenge for the widespread adoption of CCTA in this clinical context.
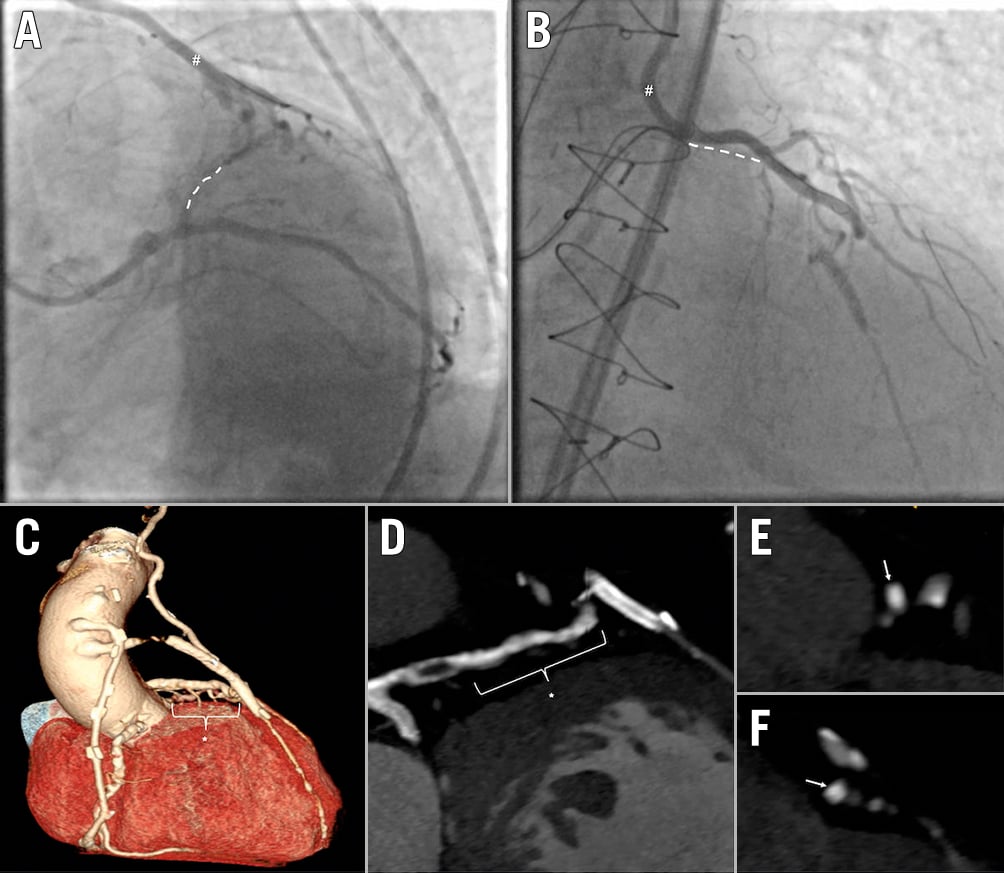
Figure 1. The role of coronary computed tomography angiography in the assessment of a heavily calcified chronic total occlusion. A,B) The coronary angiography (dual injection) of a highly symptomatic post-CABG patient with a CTO of the proximal/mid-LAD (dotted white line) and a still functioning LIMA graft (showed with “#”), insufficiently supplying the mid-distal LAD through a diffusely stenotic segment of the native diagonal branch. C) The three-dimensional computed tomographic reconstruction of the native coronary arteries (occluded LAD indicated with the asterisk), as well as the grafts. In this case, CCTA was instrumental for procedural planning, highlighting both the length and vessel course of the chronically occluded coronary segment of the LAD (D), as well as the heavy calcification throughout the occlusion. E,F) A “full moon” calcium pattern (white arrows) at the site of bifurcation with the LCx, and the connection with the diagonal branch, respectively. CABG: coronary artery bypass graft; CCTA: coronary computed tomography angiography; CTO: chronic total occlusion; LAD: left anterior descending coronary artery; LCx: left circumflex coronary artery; LIMA: left internal mammary artery
Intravascular imaging
Intraprocedural characterisation of calcium distribution is a fundamental step to guide further therapeutic strategies (Figure 2). Using intravascular imaging allows differentiation between concentric and eccentric calcium distribution. Eccentric calcification associated with calcified nodules increases the likelihood of incomplete stent expansion and poorer outcome following PCI42829. In a study by Fujii et al, intravascular ultrasound (IVUS) identified coronary calcification in 96% of patients with CTOs, while angiography-detected calcification was found in only 61%30.
The use of IVUS in CTO PCI is preferred over optical coherence tomography (OCT), because the latter requires vigorous contrast administration, which may cause expansion of coronary dissections, intramural haematoma, and worsening renal function. However, OCT has higher resolution and can be used for stent optimisation after stent deployment, if contrast administration has been limited. OCT may also be valuable for follow-up diagnostic assessments, for the detection of neoatherosclerosis, malapposition, and stent underexpansion31.
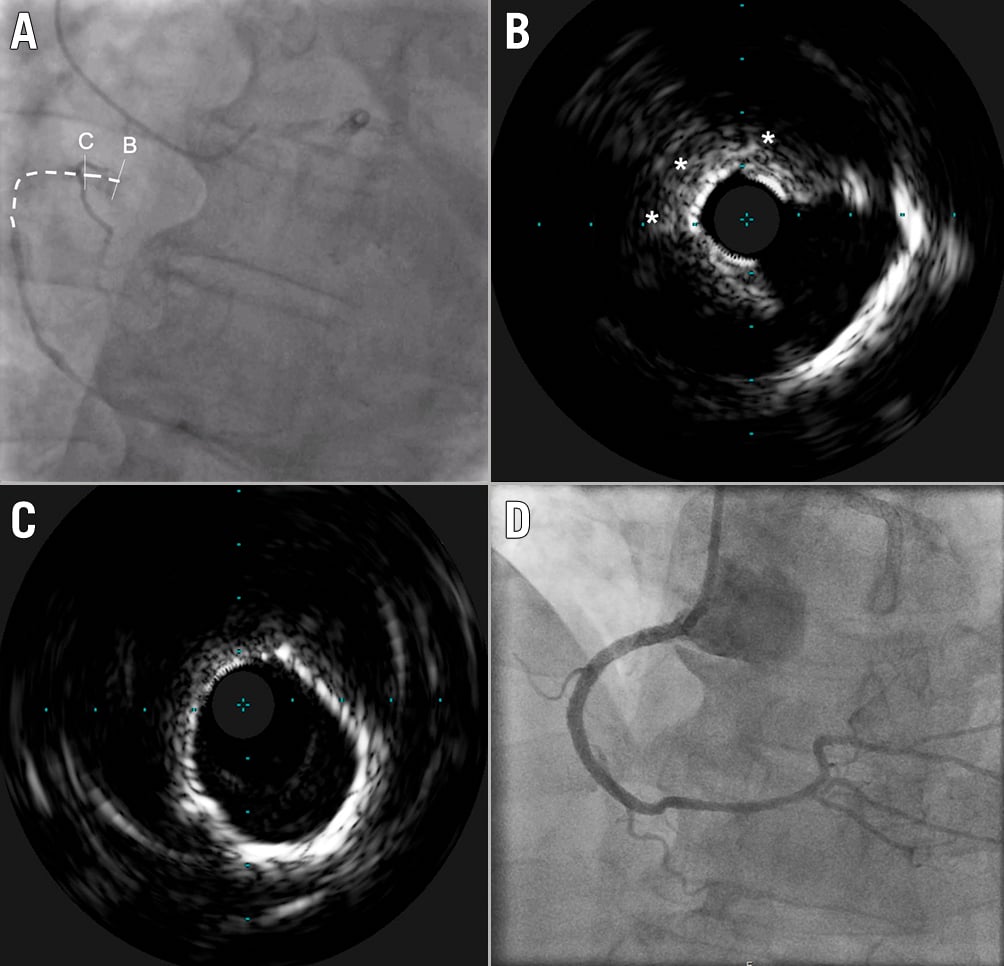
Figure 2. Intravascular ultrasound in CTO PCI. Clinical case of an ostial in-stent CTO of an RCA. A) The vessel course is shown by the dotted line. Following the wire crossing, an initial plaque modification using cutting balloon dilatation was performed in order to allow delivery of the IVUS catheter; B) IVUS assessment showed an ostial calcium nodule (asterisks), responsible for the incomplete stent expansion, that eventually led to vessel occlusion. C) Distal to the eccentric nodular lesion, the vessel remained diffusely calcified, showing a 360° arc of calcification. D) The final result, after intravascular lithotripsy with a Shockwave 4.0x12 mm balloon and stent implantation. CTO: chronic total occlusion; IVUS: intravascular ultrasound; PCI: percutaneous coronary intervention; RCA: right coronary artery
Strategy of percutaneous treatment of heavily calcified chronic total occlusions
Considering the anatomical peculiarities of heavily calcified CTOs, we propose a treatment algorithm, divided into 2 main strategical steps: (i) CTO crossing; (ii) lesion preparation (Central illustration, Figure 3).
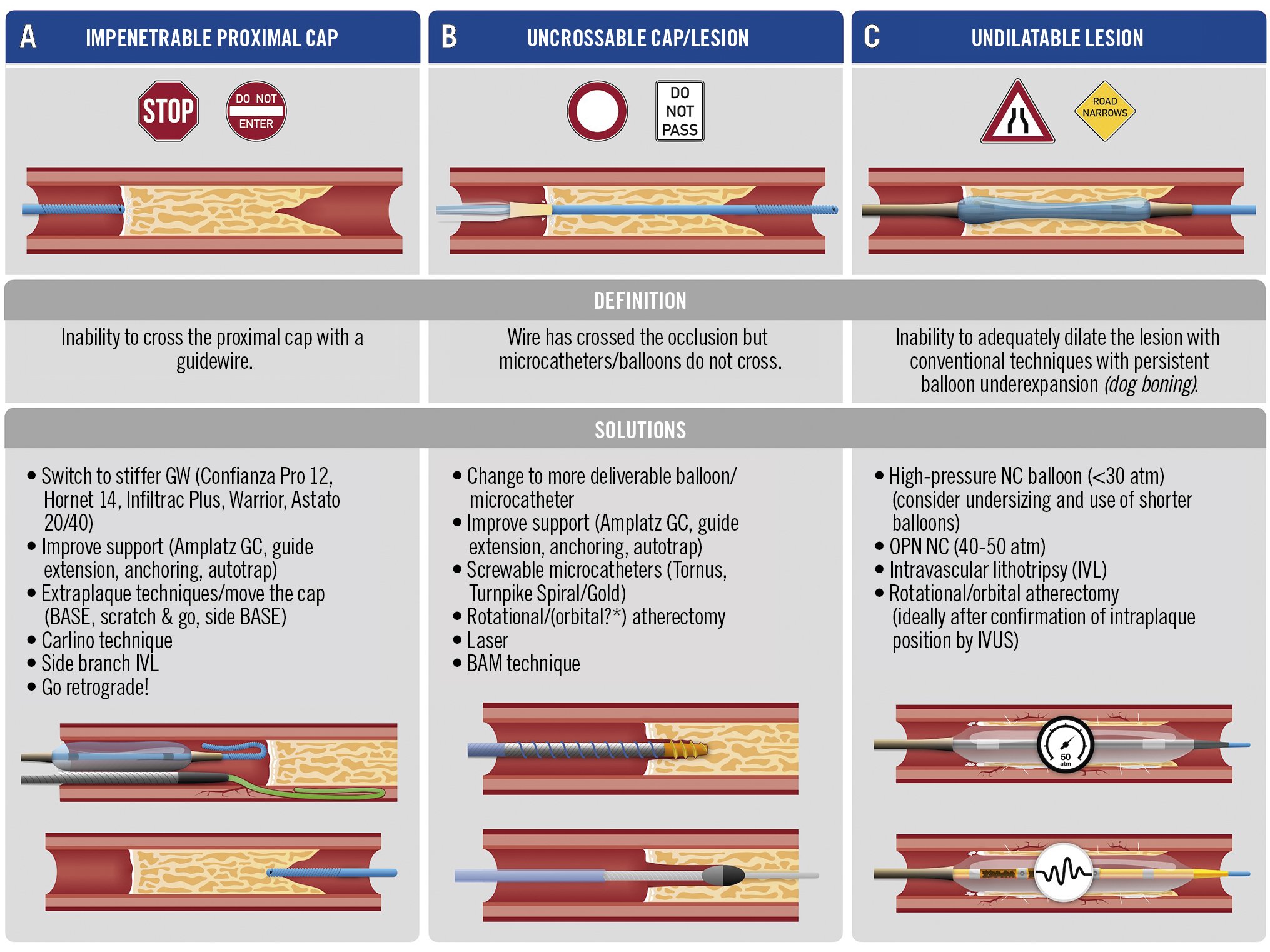
Central illustration. Percutaneous treatment strategies of heavily calcified chronic total occlusions. A) Troubleshooting options in case of an impenetrable proximal CTO cap: use of progressively stiffer guidewires; enhanced support; move-the-cap techniques (i.e., extraplaque entry proximal to the CTO lesion, resulting in extraplaque bypassing of the proximal CTO cap); the Carlino technique (selective microinjections of small amounts of contrast to disrupt the proximal CTO cap); intravascular lithotripsy; and retrograde-based strategies. B) Strategies to overcome the problem of an uncrossable cap and/or CTO lesion: use of more deliverable equipment; support enhancement; use of screwable microcatheters (active microcatheter lesion penetration); rotational/orbital atherectomy (or laser where available); use of the balloon-assisted microdissection technique (explanation in text). Of note, extraplaque techniques can also be used to resolve the problem of uncrossable caps or lesions. C) Potential solutions, in case the lesions are undilatable by conventional balloons: use of high pressure NC or very high pressure (OPN) NC balloons can be used (of note: this strategy should be performed only in cases of intraplaque tracking of the occluded segment); intravascular lithotripsy is a valid alternative to prepare undilatable lesions, in cases where an IVL balloon is deliverable. Finally, rotational/orbital atherectomy can be used to prepare calcified lesions, once the intraplaque tracking is confirmed by IVUS. *Evidence regarding orbital atherectomy is currently limited. BAM: balloon-assisted microdissection; BASE: balloon-assisted subintimal entry; CTO: chronic total occlusion; GC: guiding catheter; GW: guidewire; IVL: intravascular lithotripsy; IVUS: intravascular ultrasound; NC: non-compliant; OPN: ultrahigh pressure
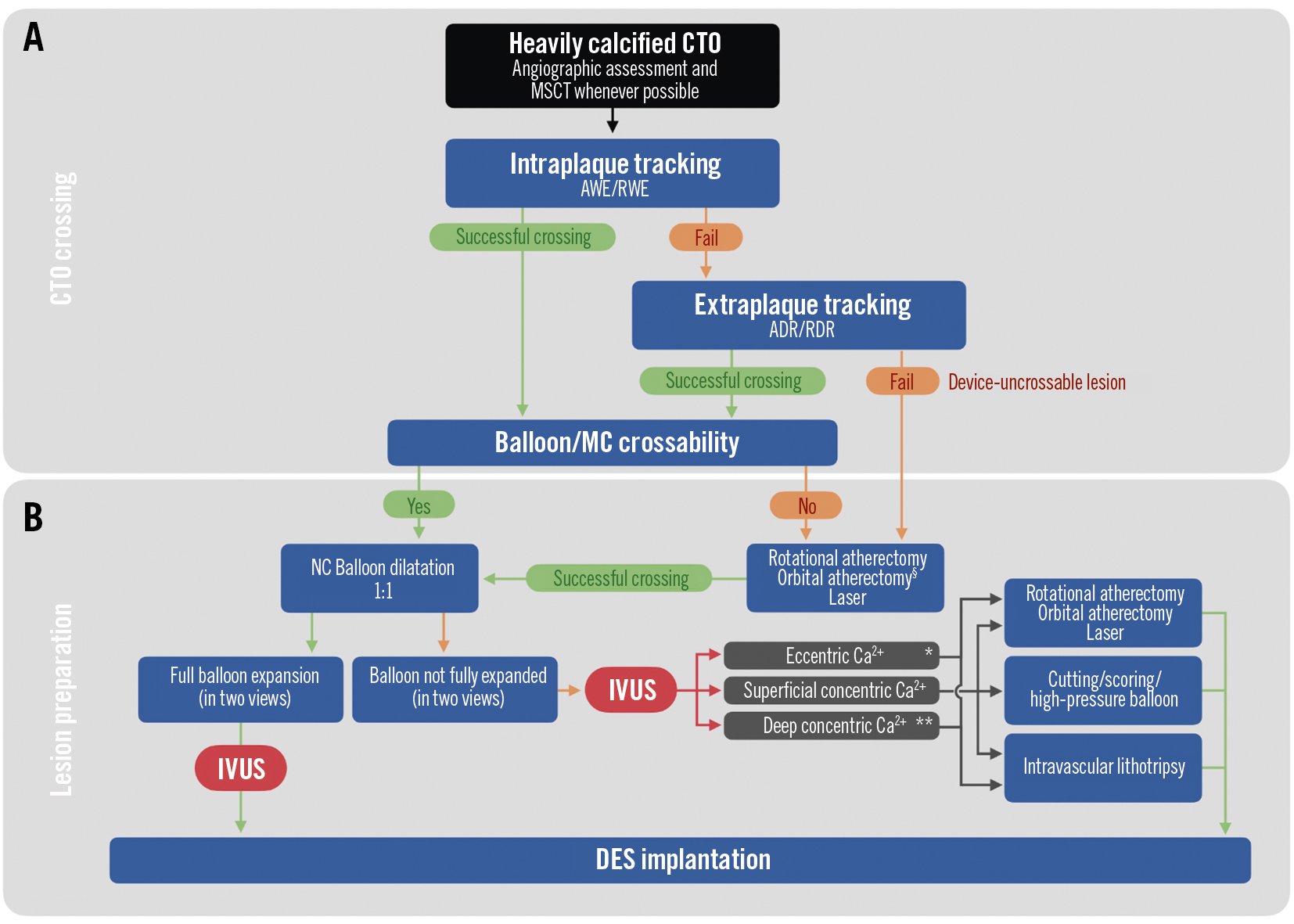
Figure 3. Algorithm for CTO crossing and lesion preparation in heavily calcified CTO. A) Crossing strategies for heavily calcified chronic total occlusions, beginning with the dual-injection angiographic assessment (and MSCT, where available). Intraplaque tracking (antegrade, retrograde) should be preferred as the initial crossing strategy; in case of failure (i.e., extraplaque tracking of the occluded segment) dissection and re-entry techniques should be used as second-line options. Once wire-based crossing is successfully performed, device (microcatheter, balloon) crossing should be attempted. In cases of device-uncrossable lesions, careful use of rotational/orbital atherectomy can be performed. B) Lesion preparation: balloon dilatation using non-compliant (NC) balloons (balloon dimension/vessel reference 1:1) should be performed to adequately prepare the lesion for stent implantation, by confirming the full expansion of the balloon in two angiographic views; IVUS should be performed before drug-eluting stent (DES) implantation. Inability of the balloon to fully expand requires precise lesion characterisation using IVUS: according to the calcium distribution characteristics, different strategies of lesion preparation can be used, as indicated. §Evidence regarding orbital atherectomy is currently limited. *Including calcific nodules. **Deep concentric calcium should be amenable to treatment, only in cases when the lumen is compromised by its presence (e.g., significant minimum luminal area reduction, incomplete balloon expansion in two views). For device-uncrossable lesions, the role of orbital atherectomy is currently not as well established as rotational or laser atherectomy. ADR: antegrade dissection re-entry; AWE: antegrade wire escalation; CTO: chronic total occlusion; IVL: intravascular lithotripsy; IVUS: intravascular ultrasound; MC: microcatheter; MSCT: multislice computed tomography; RDR: retrograde dissection re-entry; RWE: retrograde wire escalation
(i) CTO crossing with a wire
Proximal cap penetration
Penetration of a heavily calcified proximal cap can be performed using a high tip-load guidewire (Central illustration, Table 2, Figure 3). Specific microcatheters such as the Tornus (Asahi Intecc) or the Turnpike Gold (Teleflex) have a metallic tip and can be used to penetrate and disrupt the proximal heavily calcified CTO cap. Their coiled structure allows them to be “screwed” into the CTO lesion, creating a track through the calcification. The proximal CTO cap can be also modified using the balloon-assisted subintimal entry (BASE) technique (Figure 4), by creating a dissection in front of the proximal CTO cap and entering the extraplaque space using a polymer-jacketed wire in the form of a knuckle32.
Table 2. Uncrossable lesions – stepwise approach and recommendations.
| Intraplaque techniques* | Enhance guiding catheter support:- large lumen (passive support)- shape (e.g., Amplatz) (active support) |
| Use of low-profile balloons (<1.5 mm diameter) | |
| Use of guide extension | |
| Anchoring balloon | |
| “Special” microcatheters:- Tornus- Turnpike Spiral/Gold | |
| BAM | |
| Rotational/orbital/laser atherectomy | |
| Extraplaque techniques* | ADR (including BASE, side-BASE, power knuckle) |
| RDR | |
| *Intraplaque techniques are to be considered as first-line techniques, considering that the use of extraplaque techniques may subsequently limit the use of calcium preparation techniques (rotational, orbital and laser atherectomy, as well as high-pressure balloon dilatation are to be avoided, considering safety issues, when these techniques are performed in the extraplaque space). ADR: antegrade dissection re-entry; BAM: balloon-assisted microdissection; BASE: balloon-assisted subintimal entry; RDR: retrograde dissection-re-entry. | |
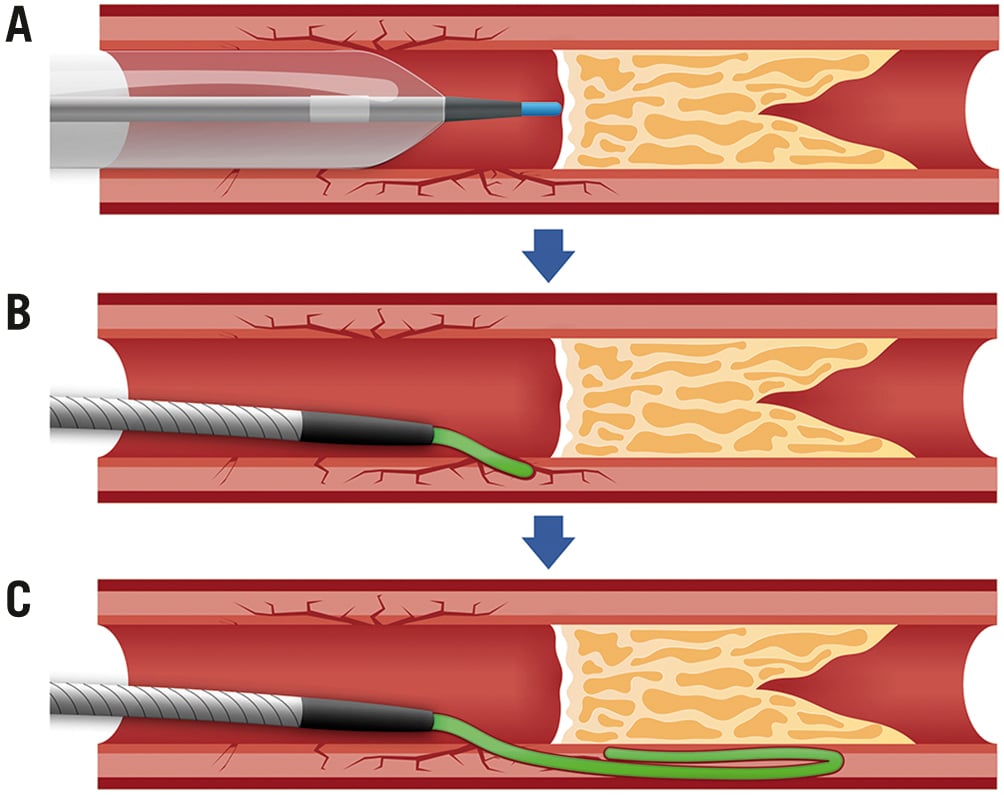
Figure 4. Step-by-step schematic representation of the balloon-assisted subintimal entry (BASE) technique. A) A semicompliant balloon, sized 1:1 to the proximal vessel reference diameter, is inflated immediately in front of the proximal CTO cap, in order to create microdissections and, hence, establish a communication between the proximal vessel lumen and the extraplaque space. B) A polymer-jacketed guidewire is inserted into the microdissections created over a microcatheter. C) Once inside the extraplaque space, the polymer-jacketed guidewire is pushed forward to form a knuckle, bypassing the heavily calcified occlusive plaque. CTO: chronic total occlusion
Intraplaque versus extraplaque tracking
After successful modification of the heavily calcified proximal cap, the occluded segment can be passed by intra- or extraplaque tracking. Intraplaque CTO tracking is almost always associated with high friction due to the advancement of equipment through the calcified segment. As such, the use of high tip-load guidewires and dedicated microcatheters, as described above, may be required throughout the occlusion. However, in cases of long and/or tortuous occluded segments, the use of polymer-jacketed guidewires, which allow extraplaque tracking whilst remaining within the vessel structure, should be preferred (Figure 5). This scenario implies bypassing the heavily calcified plaque of the CTO segment by tracking the extraplaque space33. Very high-pressure balloon inflation should be avoided in these cases, as there is a risk of non-focal, large extraplaque vessel perforations.
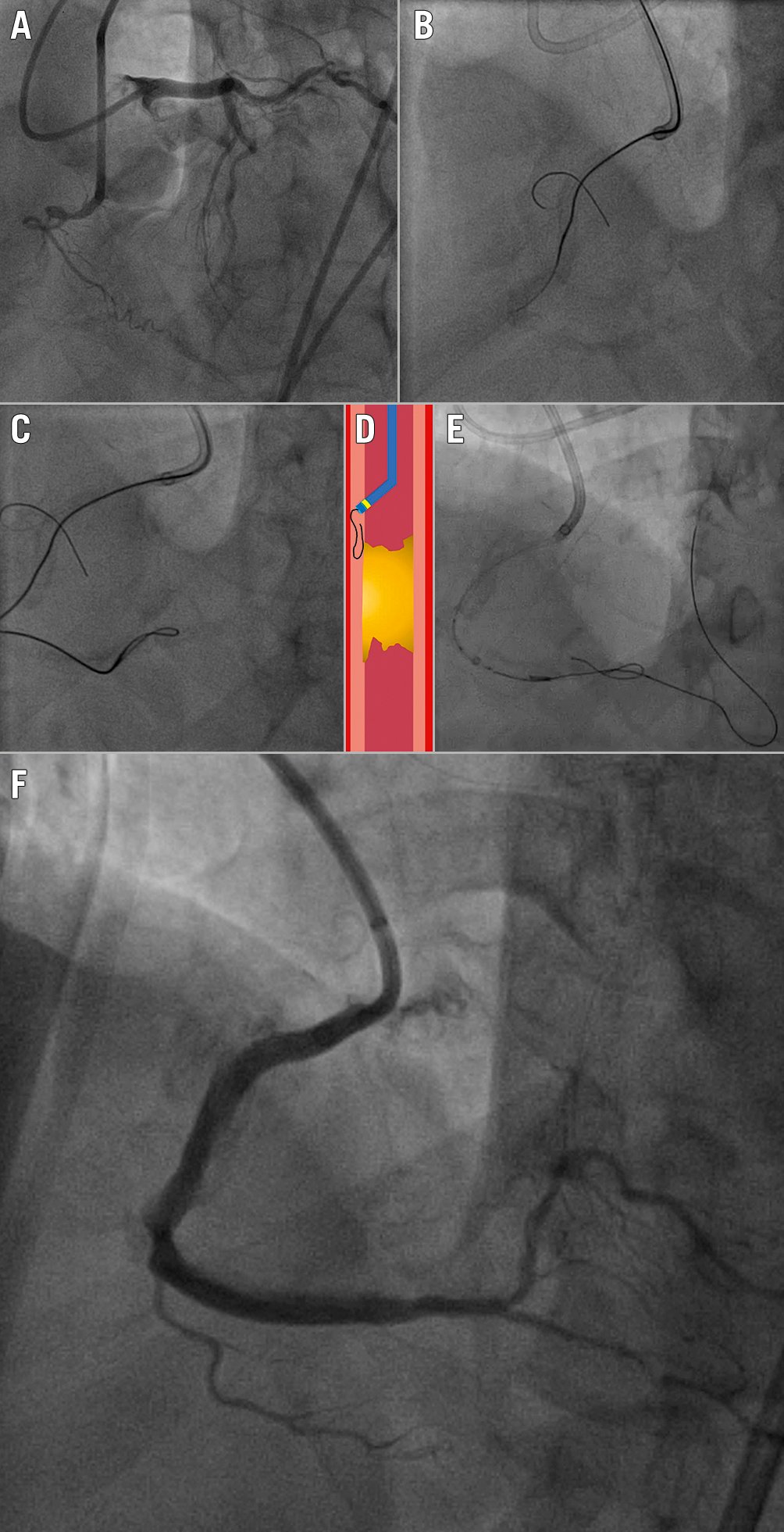
Figure 5. Crossing of a heavily calcified RCA CTO. A) Dual injection showing a CTO of the proximal RCA, expanding up to the crux. The vessel course and tortuosity are revealed angiographically to be heavily calicified. B) Initial attempt to directly wire the lesion using a polymer-jacketed wire. C) Knuckle wiring performed to “bypass” intraplaque calcification and remain within the vessel structure. D) Schematic demonstration of the knuckle-wiring technique: after achieving access to the extraplaque space with the microcatheter, a polymer-jacketed wire is pushed in an attempt to create a blunt dissection, bypassing the occlusion (shown in yellow). E) Retrograde wiring of the distal RCA allows overlapping antegrade and retrograde equipment, which is a preparation step for a reverse CART manoeuvre in an RDR strategy. F) Final result. CART: controlled antegrade and retrograde tracking; CTO: chronic total occlusion; RCA: right coronary artery; RDR: retrograde dissection-re-entry
(ii) Device-uncrossable lesion
A device-uncrossable lesion is defined as a lesion in which any low-profile balloons and/or microcatheters are unable to cross, following successful wire crossing (Table 2, Central illustration). In a multicentre series of patients treated with CTO PCI, device-uncrossable lesions were found in 9% of cases34; importantly, moderate/severe calcification was significantly more frequent (82% vs 52%), suggesting an important association between heavy calcification and the presence of device-uncrossable lesions. Several algorithms to overcome this problem have been previously proposed3536. The common denominator remains the enhancement of support and penetration, using techniques that are also used to penetrate a heavily calcified proximal CTO cap, as described above, i.e., penetrative microcatheters. In case of a primary intraplaque strategy, the wiring of a lesion using a wire intended for atherectomy can be attempted, after positioning the microcatheter as far as possible, followed by rotational or orbital atherectomy (Central illustration). Mastering antegrade and retrograde dissection-re-entry (RDR) techniques is recommended before embarking on heavily calcified CTO treatment. The use of ADR for heavily calcified CTOs is facilitated by a large distal vessel without significant calcification, in order to be able to re-enter the vessel lumen by puncturing through the calcified vascular layers37. Alternatively, the balloon-assisted microdissection (BAM) technique can be used to achieve proximal CTO cap disruption: a small (1.25-1.50 mm) balloon is engaged proximally to the CTO and inflated up to its rupture. Vo et al showed in a limited patient group that the use of the BAM technique to facilitate CTO recanalisation was effective in approximately 50% of cases33.
(iii) Calcified plaque preparation
Intravascular imaging using IVUS should be performed to characterise the calcium distribution within the CTO segment prior to plaque modification (Figure 3). Accordingly, calcific tissue can be quantified and localised. In cases of superficial, concentric calcification (intraplaque tracking), several alternatives are available to adequately prepare the lesion and allow balloon expansion: rotational/orbital atherectomy, cutting/scoring/high/very high pressure balloon dilatation, as well as intravascular lithotripsy (IVL).
Heavy calcification, alongside with proximal vessel tortuosity and lesion length, may prevent PCI equipment from reaching the CTO site. Rotational, orbital and – where available – laser atherectomy, need to be utilised in these cases before a non-compliant balloon is able to be delivered (Figure 5). Azzalini et al showed that patients undergoing rotational atherectomy during CTO PCI have similar long-term major adverse cardiac event rates as compared to other CTO patients, despite higher patient risk profiles and higher procedural complexity38.
Eccentric calcification can be more problematic to treat, particularly when extraplaque tracking occurs; in the latter case, aggressive balloon dilatation or application of other techniques can cause severe vascular trauma that can be very difficult to control, therefore we recommend not using very high dilatation pressures (<14 atm). In the case of extraplaque tracking with the CTO segment, limited use of rotational atherectomy with small burrs (<1.5 mm) at the transition zone from intra- to extraplaque can be used for plaque modification, especially to overcome proximal CTO cap resistance. Initial experience suggests that IVL is safe and effective for extraplaque calcium modification39, as well as for the treatment of eccentric calcification40. However, caution is advised, as perforations have been previously described41.
Conclusions
The treatment of heavily calcified CTOs requires a high level of expertise, considering that the use of specific tools, as well as extraplaque tracking techniques, are the rule rather than the exception. Increased calcium load adds to the already high complexity of the procedure, as severe calcification has been shown to be an independent predictor of procedural failure in most CTO scores. Histopathological studies have shown that calcium prevalence in CTO lesions is higher compared to non-CTO lesions, and this has a clinical impact, as patients with HC have lower rates of complete revascularisation and worse long-term prognoses. A preprocedural diagnostic workup using multislice computed tomography and intraprocedural in vivo characterisation by IVUS may help to identify calcium distribution in CTOs for safer and more efficient plaque modification techniques and better long-term outcomes.
Acknowledgements
We are in debt to our colleague, Dr Claire Raphael, Consultant Cardiologist, Mayo Clinic, MN, USA, for the expert English review.
Conflict of interest statement
K.Mashayekhi received consultancy fees and speaker honoraria from Abbott, Abiomed, Asahi Intecc, AstraZeneca, Biotronik, Boston Scientific, Cardinal Health, Daiichi Sankyo, Medtronic, Shockwave, Teleflex, and Terumo. S.A.Pyxaras reports proctorship and consultancy fees from Asahi Intecc and Boston Scientific; and speaker honoraria from Abiomed, AstraZeneca, and BIOTRONIK. G.S.Werner reports speaker honoraria from Asahi Intecc, BIOTRONIK, Philips/Volcano, Shockwave, Siemens Healthcare, and Terumo. C.Di Mario reports research grants from Abbott, Boston Scientific, Chiesi, Edwards Lifesciences, Medtronic, Shockwave, and Volcano. The other authors have no conflicts of interest to declare.

