Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is an alternative therapeutic option for patients with severe aortic valve stenosis (AS) and elevated surgical risk. Previous studies have suggested that the occurrence of systemic inflammatory response syndrome (SIRS) in patients undergoing TAVI is associated with an unfavourable outcome. We sought to assess the impact of different interventional access routes (transapical [TA] vs. transfemoral [TF]) and valve types (Medtronic CoreValve® [CV] vs. Edwards SAPIEN XT® [ES]) on the incidence of SIRS. In addition, the prognostic value of SIRS was evaluated.
Methods and results: Between January 2009 and July 2011 a total of 192 (out of 228) consecutive patients with severe aortic stenosis underwent TAVI at the University Hospital Frankfurt and were included in the current retrospective analysis. SIRS criteria were evaluated within the first 48 hours after TAVI. SIRS was defined according to existing definitions of the ACCP/SCCM Consensus Conference. A total of 75 (39.1%) patients developed SIRS at some time during the first 48 hours following TAVI. The occurrence of SIRS was independent from access route (TA 42.3% vs. TF 37.0%; p=0.28) as well as from type of valve used (ES 42.5% vs. CV 32.3%; p=0.11). However, the occurrence of SIRS was associated with a more than twofold higher one-year mortality rate (21.3%) compared to patients without SIRS in the first 48 hours (5.3%; p=0.04).
Conclusions: The occurrence of SIRS in the first 48 hours post procedure is associated with impaired prognosis following TAVI, but is independent from the chosen valve type and access route.
Abbreviations
AS: aortic valve stenosis
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CI: confidence interval
CPB: cardiopulmonary bypass
CRF: chronic renal failure
CV: Medtronic CoreValve®
ES: Edwards SAPIEN XT®
OR: odds ratio
SIRS : systemic inflammatory response syndrome
TA: transapical
TF: transfemoral
TAVI: transcatheter aortic valve implantation
Introduction
For decades surgical aortic valve replacement or repair was the standard therapy in patients with symptomatic aortic stenosis1. In recent years, TAVI has become established as an alternative therapeutic option for patients with severe AS and at elevated surgical risk1-3.
Although the activation of a systemic inflammatory response after cardiac surgery and CPB4-7 as well as after abdominal surgery8 is commonly accepted to be associated with an unfavourable outcome, there are limited data available concerning SIRS linked with TAVI9,10.
One recent study suggested that the occurrence of SIRS in patients undergoing TAVI is associated with an unfavourable outcome9. While this study focused only on a single valve type via the TF approach9,11, the influence of different access routes or devices on the development of SIRS remains to be elucidated.
For the first time, this study assesses the impact of different access routes (TA vs. TF) and valve types (Medtronic CoreValve®; Medtronic, Minneapolis, MN, USA, vs. Edwards SAPIEN XT®; Edwards Lifesciences, Irvine, CA, USA) on the incidence of SIRS with particular regard to the prognostic impact of SIRS.
Methods
All TAVI procedures were performed in a hybrid catheter laboratory. All medical records were systematically reviewed for pertinent medical history, diagnostic tests and interventional procedures. All clinical and procedural details as well as lab values were obtained either from interventional and/or clinical protocols or discharge summaries.
Study population
All patients with severe aortic stenosis undergoing TAVI between January 2009 and July 2011 at the medical centre of the University of Frankfurt, Germany, were screened for this study (N=228). SIRS was defined according to existing definitions of the ACCP/SCCM Consensus Conference, fulfilling at least two of the following four parameters: leukocyte count >12.0/nL or <4.0/nL, hyperventilation (respiratory rate >20 breaths per minute), tachycardia (heart rate >90 beats per minute) and temperature >38°C or <36°C (Table 1)12,13. Exclusion criteria included those with pre- or co-existing inflammation (as defined by leukocytosis/leukopaenia at admission), patients who underwent emergent or urgent open heart surgery or surgery for vascular complications (due to confounding inflammatory response induced by surgical trauma and extracorporeal circulation)9,14,15, and patients who died in the first 48 hours after TAVI (due to inability to provide data to show presence or absence of SIRS) (Figure 1). All patients received prophylactic antibiotic therapy with Cefuroxime peri- and post-interventionally for two days. No patients were lost to follow-up within the first year after performance of TAVI. The study population is also included in the registry with a total number of 426 patients published by our group in 201316.
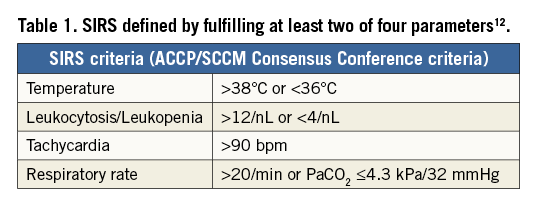
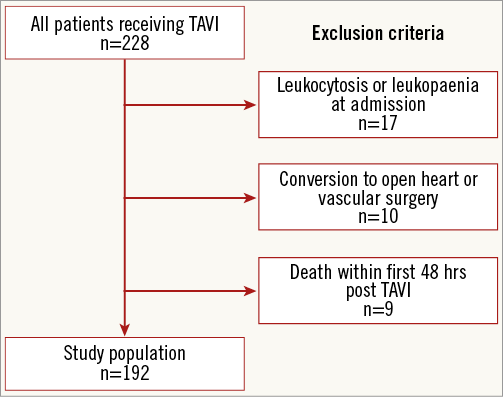
Figure 1. Study population and exclusion criteria.
Statistical analysis
Data are expressed as percentages for categorical variables and as mean±standard deviation (SD) for continuous variables. Continuous variables were compared by two-sided ANOVA test. A Cox regression analysis was performed to: 1) determine independent predictors of SIRS, and 2) determine the impact of SIRS on long-term survival (when compared to pre-specified known risk factors for mortality after TAVI). Hazard ratios (HR) and 95% confidence intervals (CIs) were calculated.
Survival was analysed using the Kaplan-Meier procedure in a time-to-event model. Survival of patients with SIRS and without SIRS was compared. Comparisons between these groups were performed by the log-rank test. Numbers of patients at risk were reported in life tables. All patients underwent follow-up (100%).
Statistical significance was assumed at p<0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
A total number of 228 consecutive patients were screened for this study. Patients a) with pre-existing inflammation (n=19), b) who underwent emergent or urgent open heart surgery or surgery for vascular complications (n=10), and c) who died in the first 48 hrs post TAVI (n=9) were excluded. In total, 192 patients were finally entered into the analysis. Of these 192 patients, 75 (39.1%) developed SIRS.
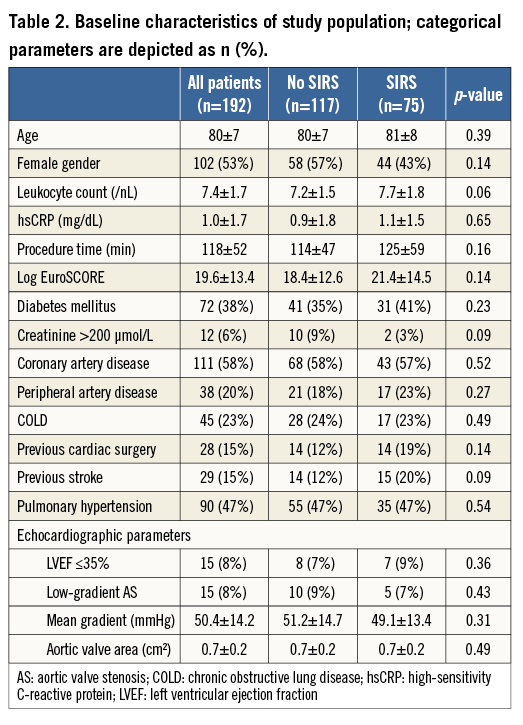
Table 2 shows the baseline clinical and echocardiographic characteristics of the study population, separated into all patients, patients who developed SIRS within 48 hrs, and patients without a SIRS diagnosis. There were equivalent numbers of male and female patients with a mean age of 80±7 years. Left ventricular ejection fraction was severely reduced in 8% of the population with no difference between the groups. Risk factors such as logistic EuroSCORE and prior cardiac surgery did not differ between groups. There was also no significant difference in the measured inflammatory markers (mean leukocyte count and mean hsCRP), procedure duration, contrast dye and chronic renal failure between groups.
VALVE TYPE AND DELIVERY APPROACH
A TF approach was chosen in most patients (n=119, 62.0%), while 71 (37.0%) patients received TAVI via the TA approach and two patients via the transsubclavian access route (1.0%). For the TF access route the CV was used in 33.9% and the ES device in 66.1% of patients. According to device description all transapical procedures were performed with the ES valve and all transsubclavian procedures with the CV.
IMPACT OF ACCESS ROUTE AND VALVE TYPE ON PREVALENCE OF SIRS
Seventy-five patients (39.1%) developed SIRS following TAVI. Of these, 21 patients (32.3%) were implanted with an ES valve, and 54 patients (42.5%) received a CV. There was no significant difference between the two valves in the occurrence of SIRS (p=0.11). In addition, SIRS was diagnosed in 44 (37.0%) of the TF patients and in 30 (42.3%) of the TA patients, respectively. Again, analysis did not present a significant difference between the two access routes (p=0.28). Due to the small number of patients receiving TAVI via the transsubclavian access route (n=2, one of whom developed SIRS) these data were not included in the final statistical access route evaluation.
PREDICTORS OF SIRS
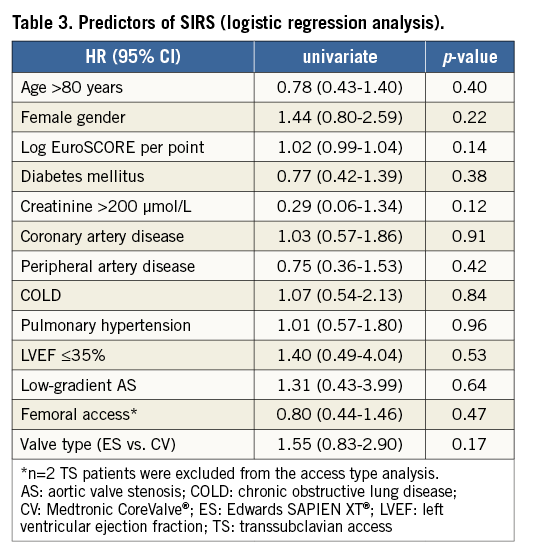
Multiple baseline and procedural variables were analysed by logistic regression to identify independent predictors of SIRS (Table 3). None of the included variables was identified as a predictor of SIRS. In particular, procedural aspects such as post-procedural aortic regurgitation have no predictive value regarding the occurrence of SIRS.
LONG-TERM OUTCOME AND PREDICTORS OF ADVERSE OUTCOME
Analysis of both groups showed no significant difference in 30-day mortality (3.4% no SIRS vs. 5.3% SIRS group; p=0.38; Table 4). However, there was a significant increase observed in one-year mortality for patients with SIRS (Figure 2).The risk of death within the first year after TAVI was almost double in patients who developed SIRS (11.1% vs. 21.3%, p=0.04; p [log-rank]=0.032.
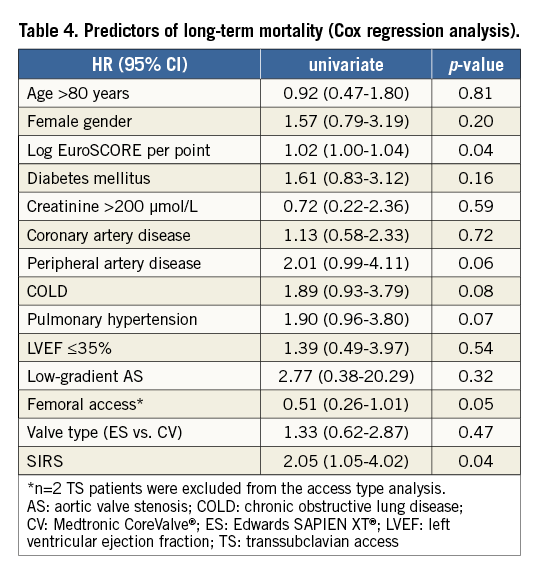
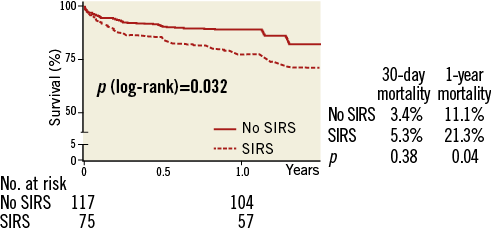
Figure 2. 30-day and one-year mortality according to the development of SIRS after TAVI.
SIRS (HR 2.05, 95% CI: 1.05-4.02; p=0.04) and logistic EuroSCORE (HR 1.02, 95% CI: 1.00-1.04; p=0.04) were both identified as univariate predictors for long-term mortality (Table 4). When analysed by Cox regression analysis, they remained independent risk factors for one-year mortality (logistic EuroSCORE HR 1.02, 95% CI: 1.00-1.04; SIRS HR 1.98, 95% CI: 1.03-3.79).
Discussion
Prior studies have shown that SIRS can be observed both after cardiac surgery5,8,14,15,17, and after TAVI10,18. In addition, Sinning et al9 demonstrated that development of SIRS after TAVI was associated with adverse outcome. After excluding several known and previously described periprocedural causes for SIRS (vascular complications and conversion to open heart surgery9), our analysis revealed a rate of 39.1% of patients developing SIRS after TAVI. This is in line with the data presented by Sinning and colleagues (prevalence of 40.1%), confirming the occurrence of a TAVI procedure-related systemic inflammatory response.
For the first time, we assessed the impact of various valve types (CV vs. ES) and interventional access routes (TA vs. TF) on the incidence of SIRS. We presumed that the increased shear stress19 from the ES balloon-expandable valve or the increased duration of rapid pacing, which may lead to SIRS through hypotension and consecutive organ hypoperfusion9, would show a bias towards one or other type of valve. Nevertheless, our data did not show a difference between the different valve types and access routes. We also presumed that, because heart surgery with or without CPB is associated with the occurrence of SIRS5,8,14,15,17, the transapical approach would arouse a higher incidence of SIRS on account of the greater invasiveness by mini-thoracotomy, pericardiotomy and puncture of the left ventricle20,21. However, our analysis did not reveal a significant difference between the TA and TF access routes. Apparently the activation of a systemic inflammatory response seems to be independent from the invasiveness of the approach. This is according to the findings of a surgical study by Diegeler and colleagues indicating that the type of operative approach does not have an impact on immune response5.
What causes SIRS in patients with TAVI? The valve type and access route did not play a role in our study. We hypothesise that this is because the main development of SIRS comes from the valvuloplasty, rapid pacing, and resultant challenging cardiac haemodynamics, not the access route or type of valve (which includes both self-expandable and balloon-expandable valves).
SIRS is associated with a significant increase in one-year mortality but not 30-day mortality. The lack of difference in short-term mortality may suggest that the adverse outcome associated with SIRS after TAVI is not derived by community-acquired or hospital-associated infectious diseases such as pneumonia or urinary tract infection, which would be estimated to lead primarily to a higher short-term mortality. Therefore, we suppose that SIRS after TAVI evaluated in this study is mostly procedure-associated. The pathomechanisms influencing long-term mortality in patients developing SIRS after TAVI remain unclear and have to be evaluated in further, appropriately designed studies. Identifying those pathomechanisms and also preceding biomarkers7,22 could be steps towards the future development of therapeutic options.
Limitations
Due to the retrospective non-randomised study design all established limitations apply. The inclusion of fewer than 200 TAVI patients might have insufficient power for a comparison between different treatment groups. Differences in baseline characteristics, which were not evaluated, cannot be excluded. In addition, there may be infectious, rheumatoid disease, or other reasons for inflammatory activation that may have led to SIRS. It would be ideal to compare these results to a population of elderly patients with aortic stenosis undergoing another invasive procedure requiring intensive care. Finally, we cannot exclude completely that SIRS occurs at least partially independently from TAVI in the course of intensive care in this mainly octogenarian population.
Conclusion
This study does not explain the pathomechanism of inflammatory response after TAVI. Therefore, further investigations are needed to identify causes and pathomechanisms of SIRS associated with TAVI in order to derive therapeutic consequences.
| Impact on daily practice Taking into account the fact that the occurrence of systemic inflammatory response syndrome (SIRS) is associated with an unfavourable outcome in patients undergoing TAVI, the main focus of attention should be in avoiding vascular complications, major bleeding and acute kidney injury known to be predictive factors for SIRS. However, the occurrence of SIRS is independent from type of valve (Medtronic CoreValve® vs. Edwards SAPIEN XT®) and access route (transapical vs. transfemoral). Therefore, these procedural features are not decision criteria in order to avoid SIRS. |
Conflict of interest statement
R. Lehmann, M. Doss and S. Fichtlscherer have received honoraria from Edwards Lifesciences (proctoring); H. Sievert has received grant support from Medtronic. The other authors have no conflicts of interest to declare.

