Abstract
Atherosclerosis is a chronic condition characterised by the build-up of plaque in the inner lining of the blood vessels and it is the main underlying cause of cardiovascular disease. The development of atherosclerosis is associated with the accumulation of cholesterol and inflammation. Although effective therapies exist to lower low-density lipoprotein cholesterol (LDL-C) levels, some patients still experience cardiovascular events due to persistent inflammation, known as residual inflammatory risk (RIR). Researchers have conducted laboratory and animal studies to investigate the measurement and targeting of the inflammatory cascade associated with atherosclerosis, which have yielded promising results. In addition to guideline-directed lifestyle modifications and optimal medical therapy focusing on reducing LDL-C levels, pharmacological interventions targeting inflammation may provide further assistance in preventing future cardiac events. This review aims to explain the mechanisms of inflammation in atherosclerosis, identifies potential biomarkers, discusses available therapeutic options and their strengths and limitations, highlights future advancements, and summarises notable clinical studies. Finally, an evaluation and management algorithm for addressing RIR is presented.
While reduction of low-density lipoprotein cholesterol (LDL-C) remains the mainstay of secondary atherosclerotic cardiovascular disease (ASCVD) treatment, the prevention and attenuation of systemic inflammation have emerged as targets of interest in patients with recurrent events1. The association between heightened systemic inflammation and increased ASCVD risk is supported by large clinical, observational and epidemiological studies. Furthermore, landmark randomised control trials exploring the use of anti-inflammatory agents to attenuate systemic inflammation have demonstrated significant reductions in secondary ASCVD events2.
Despite this evidence, preventative guidelines and commonly used ASCVD risk algorithms remain unclear regarding the clinical utility of inflammatory biomarkers in routine practice, and a significant proportion of patients continue to experience adverse cardiac events despite optimised LDL-C levels3. The significant impact of inflammation on the development of atherosclerosis implies the existence of an additional mechanism and an overlooked residual inflammatory risk (RIR). This review summarises the relevant mechanisms of atherosclerosis and provides an assessment of current increased inflammatory risk biomarkers, RIR enhancers, and therapies. Additionally, emerging therapeutic agents, future perspectives and suggested strategies are discussed.
Mechanism of atherosclerosis and inflammation
Atherosclerotic lesions, often referred to as plaques, develop within the arterial intima. Inflammation plays a pivotal role in all stages of atherosclerosis (Figure 1) and involves various immune cells in the body45. Macrophages can be categorised as M1 (proinflammatory) or M2 (anti-inflammatory) and maintaining a balance between the two is crucial for disease progression or regression67. Monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) play significant roles by recruiting monocytes and macrophages89. T and B lymphocytes also contribute to atherosclerosis, with abnormal numbers of lymphocytes being an independent risk factor. Different subsets of T cells, such as Th1, Th2, and Th17, release specific cytokines with proinflammatory or regulatory effects. CD8+ cells have a protective effect by limiting Th1 cells and macrophages. B cells regulate inflammation through interleukin (IL)-10 production, and decreased IL-10+ B cells are associated with inflammation in atherosclerosis patients1011. Neutrophils form neutrophil-extracellular-traps (NETs) and contribute to inflammation through cholesterol crystal-induced release of NETs121314. The nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome plays a crucial role in the initiation and progression of atherosclerosis by promoting vascular inflammation and interfering with lipid metabolism15. Mast cells, natural killer cells, and dendritic cells also have roles in promoting atherosclerosis through various mechanisms, including enzymatic degradation, foam cell formation, and cytokine production161718. It is also worth mentioning that inflammation and the thrombotic cascade are closely connected, where inflammation triggers and amplifies the thrombotic response, resulting in blood clot formation in specific pathological conditions.
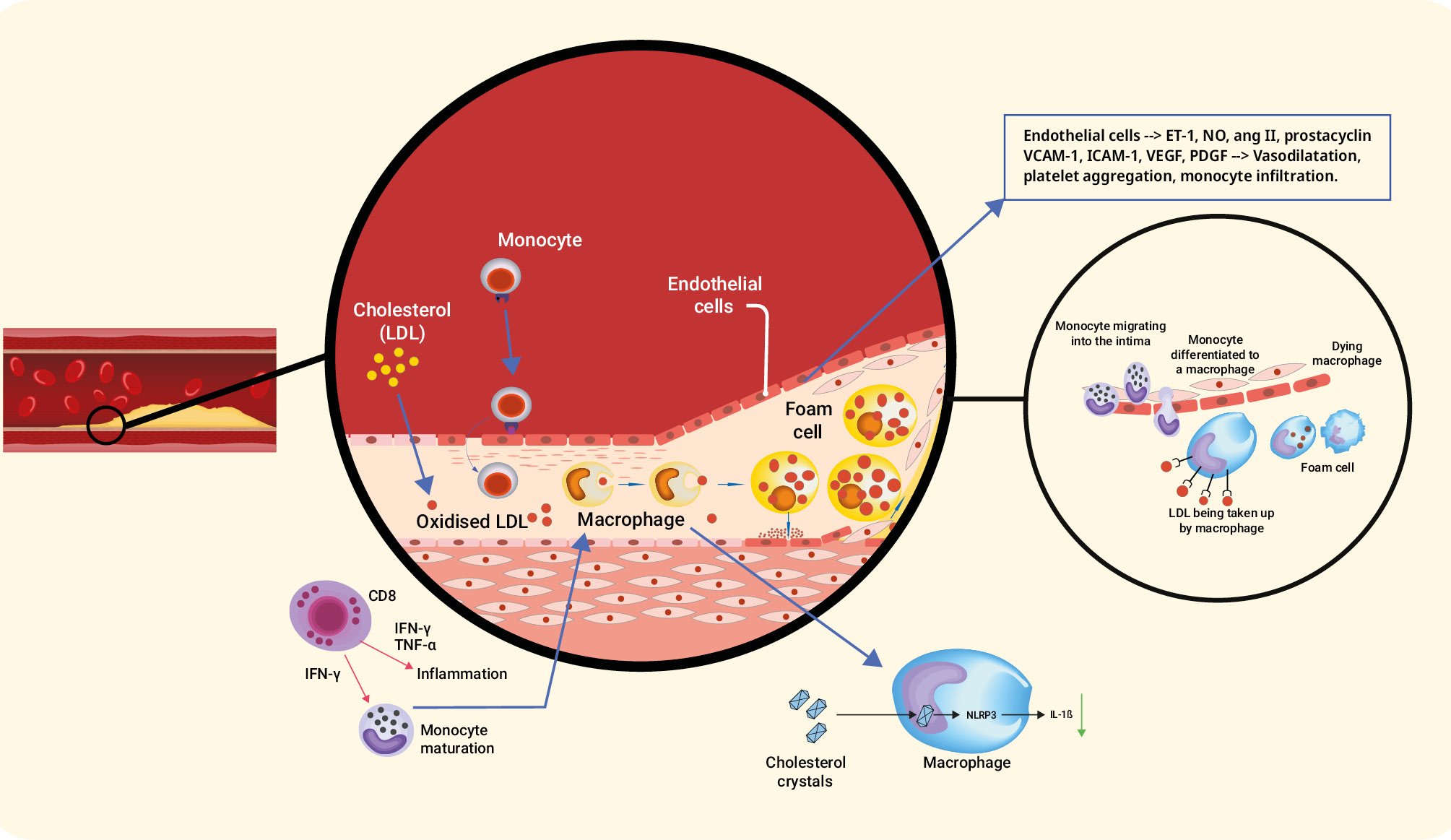
Figure 1. Mechanism of inflammation in atherosclerosis. ang II: angiotensin II; CD8: cluster of differentiation 8; ET-1: endothelin-1; ICAM-1: intercellular adhesion molecule-1; IFN: interferon; IL: interleukin; LDL: low-density lipoprotein; NLRP: nod-like receptor family pyrin; NO: nitric oxide; PDGF: platelet-derived growth factor; TNF: tumour necrosis factor; VCAM-1: vascular cell adhesion molecule-1; VEGF: vascular endothelial growth factor
Identifying inflammatory risk
Elevated biomarker levels may identify patients who could benefit from inflammation reduction therapy. Additionally, biomarkers can be used to assess treatment response. Regarding the identification of inflammatory risk, some biomarkers are currently used in clinical practice, while others are still primarily utilised in research studies (Table 1).
Table 1. Inflammatory markers.
| Biomarker | Mechanism |
|---|---|
| High-sensitivity C-reactive protein | Acute phase protein, produced by the liver |
| Its synthesis is induced by IL-1, IL-6 and TNF-α | |
| Studies usually refer to hs-CRP ≥2 mg/L as the cutoff | |
| Influenced by infection, general tissue damage, obesity, old age, hypertension, diabetes mellitus, and smoking | |
| Fibrinogen | Affects platelet aggregation, endothelial function and is a major determinant of plasma viscosity |
| Uric acid | Independent risk factor for atherosclerotic cardiovascular disease and binds atherogenic proinflammatory oxidised phospholipids which, in turn, attract inflammatory cells |
| Lipoprotein(a) | Associated with the development of hypertension, coronary artery disease, and stroke |
| Novel biomarkers | Oxidised MDA-modified LDL-C, midkine, microRNAs, pentraxins, adrenomedullin, cystatin C, Lp-PLA2, matrix metalloproteinases |
| hs-CRP: high-sensitivity C-reactive protein; IL: interleukin; LDL-C: low-density lipoprotein cholesterol; Lp-PLA2: lipoprotein-associated phospholipase A2; MDA: malondialdehyde; RNA: ribonucleic acid; TNF-α: tumour necrosis factor alpha | |
HIGH-SENSITIVITY C-REACTIVE PROTEIN
C-reactive protein (CRP) is probably the most promising biomarker and has the largest amount of investigated data. CRP is an acute-phase protein produced by the liver and it can be detected in the blood. CRP synthesis is induced by IL-1, IL-6, and tumour necrosis factor (TNF)-α. High-sensitivity CRP (hs-CRP) assays have been developed to detect minor changes in plasma CRP concentrations, and elevated levels of hs-CRP have been associated with a higher incidence of cardiovascular events. Studies usually refer to hs-CRP ≥2 mg/L as the cutoff for increased risk for cardiovascular disease19. Several cardiovascular prediction algorithms (such as the Reynolds Risk Score) showed promising results when adding hs-CRP to a clinical risk prediction model20. Very high and very low CRP values were shown to be clinically useful for risk prediction21. The JUPITER trial suggested 15 years ago that elevated hs-CRP levels, rather than other factors, are responsible for high cardiovascular event rates22. According to guidelines, hs-CRP is not currently indicated for decision-making, and there is limited information on hs-CRP distribution. The downside of this marker is the lack of specificity232425. There is no established cutoff to indicate the value which might be linked to infection rather than atherosclerosis, but the suggested values in the literature are more than 10 times higher than the 2 mg/L usually referred to26. A combined analysis from the PROMINENT, REDUCE-IT, and STRENGTH trials examined hs-CRP and LDL-C as predictors of major adverse cardiovascular events, cardiovascular death, and all-cause death27. The study concluded that, for patients taking statins, inflammation assessed by hs-CRP might be a better marker than LDL-C levels to predict the risk of future cardiovascular events and death.
OTHER INFLAMMATORY MARKERS
Fibrinogen influences clotting, platelets, and blood health and thickness, while elevated levels hinder circulation and contribute to artery hardening. Uric acid, from purine breakdown, is associated with hypertension, heart disease, and strokes, and is common in gout and kidney disease28.
Lipoprotein(a) [Lp(a)], an apolipoprotein (Apo) B-100, independently increases the risk of ASCVD by promoting atherosclerosis, inflammation, and thrombosis. Lp(a) shares structural similarities with plasminogen and tissue plasminogen activators, interfering with fibrinolysis and promoting coagulation. Additionally, Lp(a) carries cholesterol particles that contribute to atherosclerosis and binds proinflammatory oxidised phospholipids, attracting inflammatory cells and leading to smooth muscle cell proliferation29. Lp(a) holds significant relevance in assessing residual risk and warrants a dedicated review, beyond the scope of this review.
Promising novel biomarkers include oxidised LDL-C (linked to atherosclerosis)30, midkine (linked to atherosclerosis)31, specific microRNAs (associated with atherosclerosis and ischaemic events)32, pentraxins such as PTX3 (linked to inflammation and coronary stenosis)31, adrenomedullin (related to cardiovascular processes)33, cystatin C (linked to subclinical atherosclerosis)34, lipoprotein-associated phospholipase A2 (Lp-PLA2; indicative of intravascular inflammation)35, and matrix metalloproteinases, particularly stromelysin-2 (linked to atherosclerosis)36.
Inflammatory risk enhancers
Certain comorbidities independently contribute to inflammation and increased risk of ASCVD, regardless of LDL-C levels. Type 2 diabetes mellitus is a well-established example involving a complex mechanism that includes atherogenic LDL-C, hyperglycaemia, oxidative stress, and heightened inflammation37. Hypertension is another recognised cardiac risk factor, with studies supporting an independent association between inflammatory markers and hypertension, although the temporal relationship is challenging to determine38. Systemic inflammatory conditions such as systemic lupus erythematosus and rheumatoid arthritis have been investigated and were shown to carry an elevated risk of cardiovascular disease and plaque rupture39.
Smoking has been extensively studied and is known to induce endothelial dysfunction, macrophage recruitment, cytokine secretion, thrombosis, insulin resistance, dyslipidaemia, vascular inflammation, abnormal vascular growth, and angiogenesis. The Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that tobacco smoking is associated with early markers of cardiovascular injury, and smokers tend to have high levels of hs-CRP40. High or acute alcohol intake can augment nuclear factor kappa B (NF-κB) activation and TNF-α production and inhibit IL-10 levels41. A sedentary lifestyle can enhance inflammatory responses by reducing the numbers of circulating IL-10-secreting regulatory T cells and increasing proinflammatory monocytes42. Epidemiological studies have provided evidence linking air pollution to ASCVD. Inhaled small particles have been shown to contribute to endothelial dysfunction, thrombosis, vasoconstriction, and plaque instability43. Additionally, factors such as race, gender, and ethnicity play a significant role in risk enhancement44. It is often the less traditional risk factors that have the greater inflammatory-atherosclerotic predilection.
Anti-inflammatory intervention for atherosclerosis
The traditional approach of treating atherosclerosis focuses on controlling dyslipidaemia. This is usually done by lifestyle modifications and lipid-lowering medications. However, even when dyslipidaemia is well controlled, other risk factors need to be addressed. Several risk factors for atherosclerosis are non-modifiable, such as gender, race, advanced age, or family history of premature cardiovascular disease, while some are modifiable, such as diabetes mellitus, hypertension, and tobacco use. Despite optimal therapy to address the modifiable risk factors, many patients remain at increased risk of cardiac events due to persistent inflammation. This is referred to as the RIR, which could be a pharmacological target454647. This topic is vast, and we will focus on lipid-lowering and anti-inflammatory medications (Table 2). We summarise the available therapeutic options, their correlation to inflammation, and the available clinical data (Central illustration).
Table 2. Prominent anti-inflammatory interventions.
| Treatment | Mechanism |
|---|---|
| Lifestyle modifications | Fruits, vegetables, whole grains, fibre, probiotics, sleep, stress decrease and exercise have been linked to inflammation reduction by inhibiting the production of proinflammatory cytokines, limiting neutrophil migration, and positively influencing phagocytosis |
| Physical activity can stimulate anti-inflammatory responses by increasing circulating numbers of IL-10-secreting regulatory T cells and reducing circulating numbers of proinflammatory monocytes | |
| Lipid-lowering medications | |
| Statins | 1. Inhibit chemokine release and Th1-type chemokine receptors on T cells |
| 2. Inhibit NF-κB, TNF-α and IL-1β | |
| 3. Decrease the number of inflammatory cells in atherosclerotic plaques | |
| Ezetimibe | 1. Decreases macrophage content and monocyte chemoattractant protein-1 expression in atherosclerotic lesions |
| 2. Reduces the activity of NF-κB | |
| PCSK9 inhibitors | Possible decrease in stimulation and translocation of NF-κB |
| Anti-inflammatory medications | |
| Colchicine | Inhibits cytoskeletal microtubule polymerisation, leading to inhibition of vesicular secretion of inflammatory signals and preventing activation of the NLRP3 inflammasome by cholesterol crystals and reducing the release of IL-1β |
| Canakinumab | Anti-IL-1β monoclonal antibody |
| Tocilizumab | A monoclonal antibody against IL-6 receptor |
| Ziltivekimab | Antibody directed against IL-6 ligand |
| IL: interleukin; NF-κB: nuclear factor kappa B; NLRP3: nod-like receptor family pyrin domain containing 3 inflammasome; PCSK9: proprotein convertase subtilisin/kexin type 9; TNF: tumour necrosis factor | |
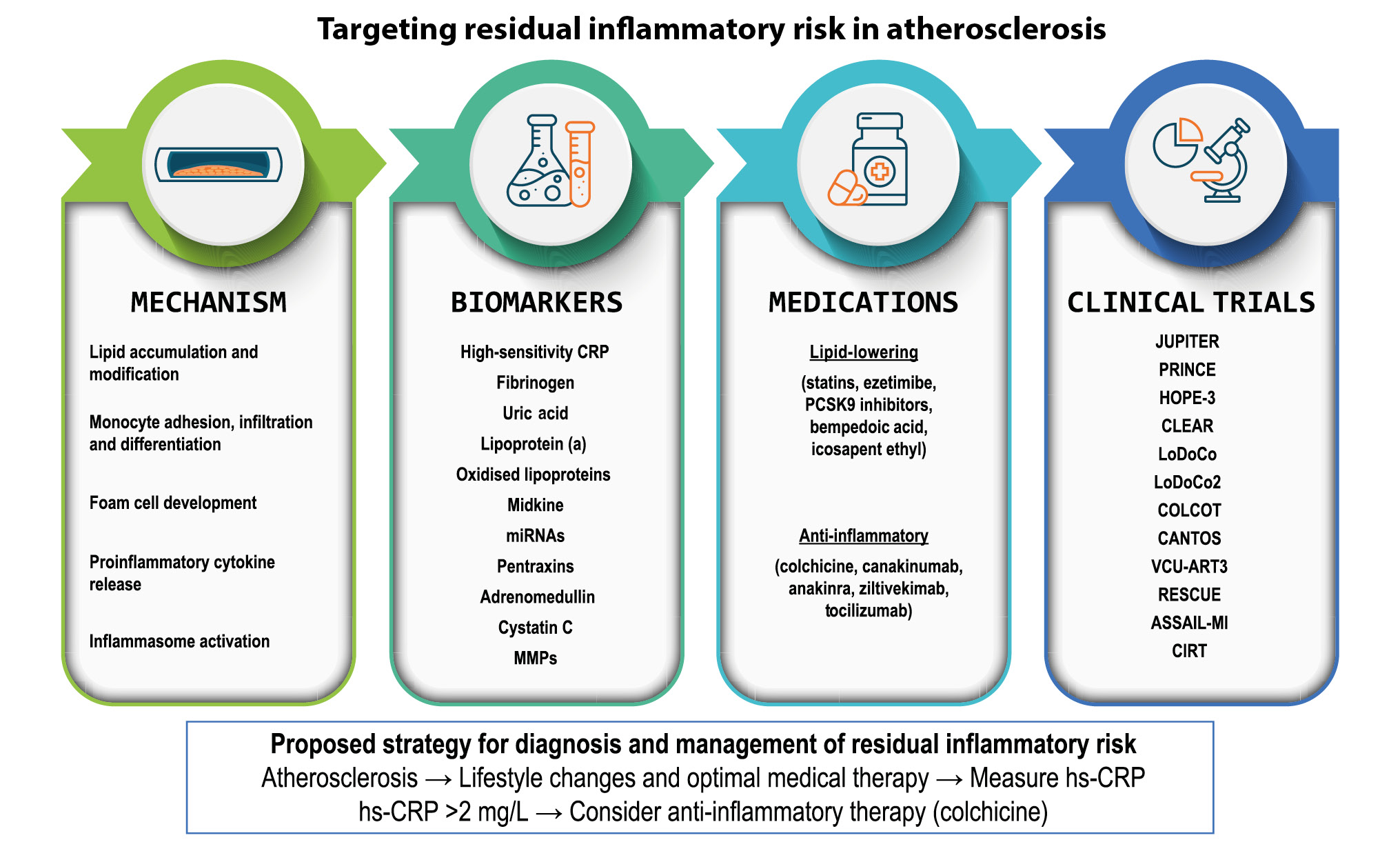
Central illustration. Mechanisms of inflammation in atherosclerosis, biomarkers of inflammation in atherosclerosis, potential treatments, and prominent clinical trials. hs-CRP: high sensitivity C-reactive protein; IL: interleukin; miRNA: microRNA; MMPs: matrix metalloproteinases; PCSK9: proprotein convertase subtilisin/kexin type 9
Lifestyle modification
Lifestyle management − focusing on diet, smoking cessation, and exercise − is key in preventing ASCVD. Recommended diets include those that are low in saturated fats, sugar, alcohol, and sodium and high in polyunsaturated fats, potassium, vitamins, and fibre. Weight loss alone may not significantly reduce atherosclerosis, so exploring antioxidant-rich foods is worthwhile. While dietary regimens have limited impact on LDL-C reduction, the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diets have anti-inflammatory effects4849. Specific elements such as alpha-lipoic acid, arachidonic acid50, resolvins51, and long-chain n-3 polyunsaturated fatty acids can reduce inflammation52. Physical activity also reduces inflammation and positively affects atherosclerosis and cardiac health53. Smoking cessation is a well-established lifestyle change for ASCVD prevention54.
Lipid-lowering drugs
STATINS
Statins are also known as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors and have long been used as a treatment for atherosclerosis55. The mechanism of this class of medications is to inhibit HMG-CoA reductase and block the intracellular mevalonic acid metabolism pathway, reducing intracellular cholesterol synthesis. This leads to an increase in the number and activity of LDL-C receptors on the cell membrane surface, which clears serum cholesterol.
Statins are mainly used in the treatment of atherosclerosis due to their cholesterol-lowering action, which not only reduces total cholesterol (TC) and LDL levels but also reduces triglyceride (TG) levels and improves high-density lipoprotein (HDL) levels. In addition, statins have anti-inflammatory properties, which are exhibited by a reduction in the release of C-reactive peptide, chemokines, cytokines, and adhesion molecules and modulation of T-cell activity. Statins inhibit the migration of leukocytes due to a decrease in the expression of adhesion molecules ICAM-1, lymphocyte function-associated antigen-1, and monocyte chemotactic protein-1; they also inhibit NF-κB, TNF-α, and IL-1β, thus decreasing the number of inflammatory cells in atherosclerotic plaques56.
The relationship between statin therapy and hs-CRP is important when discussing RIR. In the PROVE-IT TIMI 22 study, subjects who achieved hs-CRP levels <2 mg/L had fewer cardiovascular events, independent of LDL-C reduction57. The combination of simvastatin and ezetimibe yielded comparable results in the IMPROVE-IT study58. In the JUPITER study, the magnitude of hs-CRP reduction achieved with rosuvastatin was proportional to the reduction in cardiovascular risk22. Statins may be categorised by intensity and dose59. Zhang et al published a meta-analysis examining CRP levels in various types and doses of statins and showed that simvastatin 40 mg/day might be the most effective therapy; atorvastatin 80 mg/day showed the best long-term effect60. The Pravastatin Inflammation/CRP Evaluation (PRINCE) trial demonstrated that pravastatin 40 mg/day significantly reduced plasma CRP levels independent of any changes in LDL-C levels, and the HOPE-3 (Heart Outcomes Prevention Evaluation-3) study on intermediate-risk participants without cardiovascular disease supported the hs-CRP-lowering effect of rosuvastatin regardless of CRP and lipid levels at baseline5661.
EZETIMIBE
Ezetimibe inhibits intestinal cholesterol absorption by selectively blocking the Niemann-Pick C1-Like 1 (NPC1L1) protein in the jejunal brush border, affecting the uptake of intestinal lumen micelles into the enterocyte. Ezetimibe might decrease macrophage content and monocyte chemoattractant protein-1 expression62. Morrone et al reported higher levels of hs-CRP reduction with a combination therapy of ezetimibe and statins than for monotherapy with statins63.
PROPROTEIN CONVERTASE SUBTILISIN/KEXIN TYPE 9 INHIBITORS
Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to LDL receptors and targets them for lysosomal degradation. PCSK9 inhibitors (PCSK9-i) prevent this process, and the first two types of PCSK9-i approved by the U.S. Food and Drug Administration (FDA) were alirocumab and evolocumab (both monoclonal antibodies). These therapies are recommended for patients with high LDL-C levels despite statin therapy and to treat patients who cannot tolerate statins because of adverse side effects. A relatively new approach to block PCSK9 synthesis includes inclisiran, a double-stranded small-interfering RNA that specifically targets and induces PCSK9 messenger RNA (mRNA) degradation. PCSK9-i have shown a significant reduction in cardiovascular events in clinical trials64.
An analysis from the FOURIER trial showed that LDL-C reduction with evolocumab reduces cardiovascular events across all hs-CRP strata, with greater absolute risk reductions in patients with higher baseline hs-CRP levels65. However, in a post hoc analysis of the SPIRE trials in a stable outpatient population, evidence of residual inflammatory risk persisted among patients treated with both statin therapy and PCSK9-i66. Animal models have shown PCSK9 to have structural homology to a domain on resistin, an adipocyte-specific hormone, which manages proinflammatory stimulation and nuclear translocation of NFâкB, and this might add anti-inflammatory properties67. Rosenson and Goonewardena revealed immune-modulating properties of this group of medications by profiling immune cells and examining specific changes68. PCSK9-i have been shown to reduce monocyte levels of intracellular lipids and improve monocyte phenotypes with decreased monocyte migration69. This contradicts clinical trials that have not shown changes in inflammatory markers, suggesting that ASCVD event reduction by PCSK9-i is solely because of LDL-C lowering. Another important study to highlight is the PACMAN-AMI trial, which demonstrated that, in patients with acute myocardial infarction, the inclusion of subcutaneous biweekly alirocumab, alongside high-intensity statin therapy, led to significantly greater coronary plaque regression in non-infarct-related arteries after 52 weeks when compared to the placebo group70.
OTHER LIPID-LOWERING THERAPIES
Bempedoic acid (BA) reduces LDL-C and hs-CRP, similarly to statins, potentially addressing both inflammatory and cholesterol risks without replacing statins71. Lomitapide and mipomersen have potential anti-inflammatory effects for familial hypercholesterolaemia7273. Icosapent ethyl, used for severe hypertriglyceridaemia and ASCVD, may have anti-inflammatory properties by inhibiting IL-1β and IL-674. HDL may have anti-inflammatory properties, reversing LDL-C effects, and high hs-CRP levels are linked to HDL dysfunction75.
Targeting inflammatory pathways
The ongoing research in this rapidly evolving field continues to identify potential new medications and biomarkers. We will focus on known medications and relevant prominent studies with significant clinical outcomes (Table 3).
Table 3. Summary of prominent clinical trials.
| Name of study | Medication | Inflammatory results | Clinical outcomes |
|---|---|---|---|
| CANTOS2 (2017) | canakinumab | Reduced hs-CRP values compared to baseline and placebo | Patients with hs-CRP levels that dropped below 2 mg/L had a significant 25% reduction in major events |
| VCU-ART3 83 (2020) | anakinra | Hs-CRP was significantly lower in patients receiving anakinra versus placebo | The incidence of death or new-onset heart failure or of death and hospitalisation for heart failure was significantly lower with anakinra versus placebo |
| ASSAIL-MI 106 (2021) | tocilizumab | A significant difference was seen in hs-CRP area under the curve in tocilizumab patients compared to placebo | There was a 21% difference between the final infarct size at 6 months in the tocilizumab and placebo groups, but this difference was not statistically significant |
| RESCUE 84 (2021) | ziltivekimab | Median hs-CRP levels were significantly reduced compared to the placebo group | The medication was well tolerated, did not affect the total cholesterol to HDL cholesterol ratio, and there were no serious injection-site reactions |
| LoDoCo 78 (2013) | colchicine | N/A | Colchicine 0.5 mg/day administered in addition to statins and other standard secondary prevention therapies appeared effective for the prevention of cardiovascular events in patients with stable coronary disease |
| LoDoCo2 79 (2020) | colchicine | N/A | A composite of cardiovascular death, myocardial infarction, ischaemic stroke, or ischaemia-driven coronary revascularisation occurred less in patients taking colchicine |
| COLCOT 80 (2019) | colchicine | N/A | Time to first cardiovascular event in MI patients was better in patients on colchicine versus placebo |
| Combined analysis from the PROMINENT, REDUCE-IT, and STRENGTH trials 27 (2023) | - | Hs-CRP reduction was significantly associated with major adverse cardiovascular events | Inflammation-assessed hs-CRP might be a better marker than LDL-C levels to predict the risk of future cardiovascular events and death |
| HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; N/A: not applicable | |||
COLCHICINE
Colchicine works by inhibiting cytoskeletal microtubule polymerisation, leading to the inhibition of vesicular secretion of inflammatory signals and preventing activation of the NLRP3 inflammasome by cholesterol crystals and reducing the release of IL-1β76. In animal models, therapy with colchicine showed lower plasma CRP levels than therapy with atorvastatin, and a pilot study of 200 patients with clinically stable coronary artery disease (CAD) already receiving atorvastatin showed that 4 weeks of colchicine therapy led to a 60% decrease in hs-CRP levels77.
The LoDoCo Trial (The effect of low dose colchicine on the natural history of patients with stable CAD) was a prospective, randomised, observer-blinded endpoint trial. A total of 532 patients with stable CAD receiving aspirin and/or clopidogrel and statins were randomly assigned colchicine 0.5 mg/day or no medication and were followed for a median of 3 years78. The primary outcome was defined as the composite incidence of acute coronary syndrome (ACS), out-of-hospital cardiac arrest, or ischaemic stroke. The primary outcome occurred in 5.3% of patients who received colchicine and in 16.0% of those who did not. In a secondary analysis that excluded 32 patients assigned to colchicine who withdrew within 30 days because of intestinal intolerance and 7 patients who did not start treatment, the primary outcome occurred in 4.5% versus 16.0% (p<0.001). The study concluded that colchicine 0.5 mg/day administered in addition to statins and other standard secondary prevention therapies appeared effective for the prevention of cardiovascular events in patients with stable coronary disease.
The LoDoCo2 Study was an investigator-initiated, double-blind, placebo-controlled trial that randomised 5,552 patients who had chronic coronary disease to take either colchicine 0.5 mg daily or a placebo79. The primary endpoint was defined as a composite of cardiovascular death, myocardial infarction, ischaemic stroke, or ischaemic-driven coronary revascularisation. The median follow-up was about 30 months. The primary endpoint occurred in 6.8% of patients in the colchicine group and 9.6% of patients in the placebo group (p<0.001). When the components of the primary endpoint were analysed separately, a consistent trend was seen with all endpoints, and myocardial infarction and ischaemia-driven coronary revascularisation were both significantly less frequent in the colchicine group. In this study, more than 90% of patients tolerated colchicine, with no significant side effects. The study concluded that colchicine might be a good choice for long-term prevention of cardiovascular events in patients with chronic coronary disease.
The Colchicine Cardiovascular Outcomes Trial (COLCOT) included a total of 4,745 patients who had experienced a myocardial infarction (MI) within the previous 30 days and were randomised to 0.5 mg colchicine or placebo (patients with stable coronary artery disease were not included in COLCOT, unlike in the LoDoCo trials). The primary composite endpoint was defined as the time to first event of one of the following: cardiovascular death, cardiac arrest, MI, stroke, or urgent hospitalisation for angina requiring coronary revascularisation80. All patients received guideline-directed medical therapy and percutaneous coronary intervention (PCI), if indicated, before randomisation. The follow-up length was only 23 months. The rate of the primary composite endpoint was significantly lower in the colchicine group than in the placebo group (5.1% vs 7.1%; p=0.004). Other adverse events were similar between the two groups except for nausea, flatulence, and pneumonia, all of which had rates that were significantly higher in the colchicine arm. The study implied that colchicine effectively reduces the risk of first ischaemic cardiovascular events compared to a placebo. The recently published MACT Pilot Study results focused exclusively on individuals with ACS undergoing PCI. The findings revealed that discontinuing aspirin one day after PCI and administering low-dose colchicine, alongside either ticagrelor or prasugrel, is not only safe but also linked to beneficial effects on platelet function and inflammatory profiles81.
INTERLEUKINS AND CHEMOKINE INHIBITORS
Canakinumab is a human anti-IL-1β monoclonal antibody, and its mode of action is based on the neutralisation of 1β signalling, resulting in the suppression of inflammation. This medication has mostly been used for the treatment of autoimmune disorders.
The Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) was the first study to prove the benefit of targeting inflammation (published in 2017). The study evaluated the usage of three different doses of canakinumab (50 mg, 150 mg, and 300 mg) compared to placebo; the drug was administered subcutaneously every three months2. The primary endpoint of cardiovascular events, defined as non-fatal MI, non-fatal stroke, and cardiovascular death, was evaluated at 24 months. Over 17,000 patients were screened from 39 countries, with 10,061 patients successfully randomised. At 24 months, all three canakinumab arms showed a significant reduction in hs-CRP values as compared to baseline and the placebo arm. Both the 150 mg and 300 mg arms demonstrated a significant reduction in cardiovascular events as compared to the placebo arm. When combining the 150 mg and 300 mg arms together in comparison to the placebo arm, patients receiving canakinumab demonstrated a 39% relative risk reduction in hs-CRP values and a 15% relative risk reduction in the primary endpoint. Additionally, when looking at patients with hs-CRP levels above and below the median at enrolment, those with a higher hs-CRP at baseline benefitted more.
A subanalysis of the study looked at the group of CANTOS patients who achieved hs-CRP levels below 2 mg/L and compared them with subjects who had smaller reductions82. The reduction in hs-CRP after a single dose of canakinumab was a strong predictor of canakinumab efficacy. Those with hs-CRP levels that dropped below 2 mg/L had a significant 25% reduction in major events (p<0.0001). This finding suggests that a goal of less than 2 mg/L of hs-CRP is needed to adequately reduce RIR. The major side effect of canakinumab in CANTOS was a small increase in fatal infections. Criticism was mainly directed at the lack of a clear dose response, the increased risk for a fatal complication due to infection, the need for an injection, and the high cost of the medication.
Another interleukin inhibitor is anakinra, which is an IL-1 receptor antagonist. The Virginia Commonwealth University Anakinra Remodeling Trial 3 (VCU-ART3) was a randomised, placeboâcontrolled, doubleâblind, clinical trial that included 99 patients with ST-elevation myocardial infarction (STEMI)83. The patients were assigned to 2 weeks of treatment with either anakinra once daily, anakinra twice daily, or placebo. The levels of hs-CRP were significantly lower in patients receiving anakinra versus placebo. The incidences of death or newâonset heart failure or of death and hospitalisation for heart failure were significantly lower with anakinra versus placebo (9.4% vs 25.7%; p=0.046 and 0% vs 11.4%; p=0.011). The incidence of serious infection was not different between the anakinra and placebo groups. Injection site reactions occurred more often in patients receiving anakinra versus placebo. The study concluded that in STEMI patients, an anti-inflammatory medication such as anakinra might reduce the inflammatory response and, thus, lead to better outcomes.
Ziltivekimab is a novel human antibody directed against the IL-6 ligand. The Trial to Evaluate Reduction in Inflammation in Patients with Advanced Chronic Renal Disease Utilizing Antibody Mediated IL-6 Inhibition (RESCUE) was a randomised, double-blind, phase 2 trial of participants (n=264) with moderate to severe chronic kidney disease and high-sensitivity CRP levels of at least 2 mg/L84. Subjects were randomly allocated to subcutaneous administration of placebo or ziltivekimab (at different doses) every 4 weeks for 24 weeks. The study compared the change in hs-CRP levels after 12 weeks of treatment as the primary outcome. At 12 weeks, median hs-CRP levels were reduced by 77% for the 7.5 mg group, 88% for the 15 mg group, and 92% for the 30 mg group, compared with 4% for the placebo group. Dose-dependent reductions were also seen for inflammatory markers. The medication was well tolerated, and there were no serious side effects. This study’s outcomes are extremely strong, with considerable prognostic potential, and this IL-6 inhibitor might be considered as effective as canakinumab.
Tocilizumab and sarilumab are both monoclonal antibodies against the IL-6 receptors. Endothelial function was shown to be improved by tocilizumab in patients at high risk for CAD, despite increasing LDL-C. The trial ASSessing the Effect of Anti-IL-6 Treatment in Myocardial Infarction (ASSAIL-MI) was a double-blind, placebo-controlled, randomised study in patients with acute STEMI8586. The participants were randomised to receive either a single infusion of 280 mg tocilizumab or placebo. The primary endpoint was the myocardial salvage index, which was measured by magnetic resonance imaging 3 to 7 days post-event. The tocilizumab group had higher myocardial salvage index scores, and a significant difference was seen in the CRP area under the curve, at a median of 1.9 mg/L/h (interquartile range [IQR] 0.9 to 4.9) in the tocilizumab patients compared to 8.6 mg/L/h (IQR 5.0 to 17.9) in the placebo group (p<0.001). There was a 21% difference between the final infarct size at 6 months in the tocilizumab and placebo groups, but this difference was not statistically significant (p=0.08). There was no difference in serious events (MI, coronary artery bypass grafting, subarachnoid haemorrhage, resuscitated ventricular fibrillation, ventricular tachycardia, chest pain, and ischaemic stroke) between the two groups (p=0.57).
MLN1202, a neutralising monoclonal antibody against CCR2, led to a notable reduction in hs-CRP, compared to placebo, in a preliminary study87.
METHOTREXATE
Methotrexate inhibits lymphocyte proliferation and inflammatory cytokine production via adenosine binding with the A2 receptor. The Cardiovascular Inflammation Reduction Trial (CIRT) was a randomised, double-blind trial of low-dose (15-20 mg weekly) methotrexate or placebo in 4,786 patients with earlier MI or multivessel coronary disease who had either type 2 diabetes or metabolic syndrome88. This anti-inflammatory agent, which is available in a generic form, is considered safe at low doses. Methotrexate had no impact on the primary endpoint of major adverse cardiovascular events, compared with placebo. This was true for the original composite of non-fatal MI, non-fatal stroke, or cardiovascular death and for the final endpoint, which added hospitalisation for unstable angina leading to urgent revascularisation. Mortality was higher with low-dose methotrexate, both from cardiovascular causes and overall, and this was why the trial was stopped after a median follow-up of 2.3 years. Methotrexate did not reduce levels of CRP, IL-1β, or IL-6.
HYDROXYCHLOROQUINE, ALLOPURINOL, AND SALICYLATES
Hydroxychloroquine is used for inflammatory rheumatic diseases, altering lysosomal pH and reducing inflammation by inhibiting TLR7 and TLR989. Allopurinol treats hyperuricaemia in gout, potentially reducing atherosclerosis-related inflammation in mice, but its clinical effectiveness remains inconclusive90. Salicylates inhibit NF-κB activity but did not reduce inflammation in atherosclerosis patients in the TINSAL-T2D and TINSAL-FMD trials91.
OTHER MEDICATIONS UNDER INVESTIGATION
Dapansutrile, an NLRP3 inflammasome inhibitor, had no significant effect on CRP levels92. Succinobucol, an antioxidant, did not reduce cardiovascular events in a phase III trial93. Darapladib, an Lp-PLA2 inhibitor, showed conflicting results in clinical studies94. Losmapimod (p38 inhibitor) and inclacumab (P-selectin inhibitor) are currently being investigated. Antithrombotic medications in CAD patients may offer additional benefits beyond their primary purpose due to the relationship between inflammation and thrombosis95.
Strategy: assessment and treatment
The presence of local and systemic inflammation in patients with atherosclerosis is well established. Targeted reduction of inflammation is crucial for preventing cardiovascular events in patients already receiving optimal medical therapy. Biomarkers are available to detect and measure the inflammation process, with hs-CRP being the most widely investigated and accessible biomarker for this purpose. Several anti-inflammatory medications, including colchicine, have shown improvement in both inflammation and clinical outcomes in cardiac patients. Recently, colchicine was even widely approved for this purpose by the FDA96.
To tailor therapies effectively, clinicians need to evaluate the overall risk/benefit ratio for their patients. There are two main patient phenotypes: those with residual cholesterol risk and elevated LDL-C levels despite medical therapy and those with RIR. The first group should receive additional LDL-C-lowering treatment according to guidelines, such as the European Society of Cardiology (ESC) or American College of Cardiology/American Heart Association recommendations [49]. The second group consists of patients with LDL-C at target levels but still experiencing RIR. While ESC guidelines suggest lowering LDL-C to less than 40 mg/dL or adding colchicine for patients with recurrent cardiac events despite optimal medical therapy, these indications are primarily based on secondary prevention after an event has occurred.
The data presented in this review support using a biomarker to diagnose RIR and treat inflammation proactively before an event occurs. Non-invasive or invasive imaging techniques, such as coronary computed tomography, carotid ultrasound, positron emission tomography, intravascular ultrasound, near-infrared spectroscopy, and optical coherence tomography, can show atherosclerotic arteries, while risk scores can identify patients at high risk of atherosclerosis even without visual evidence of plaque. Once a patient reaches the target LDL-C level, we propose using a biomarker to assess the presence of RIR rather than adopting a watchful waiting approach for a new event (Figure 1).
Hs-CRP levels have been proven to independently predict cardiovascular events (both new and recurrent) in multiple, prospective epidemiological cohorts. Stable hs-CRP levels over time, excluding acute infection, are typically below the commonly used cutoff value of 2 mg/L. If a patient has elevated hs-CRP levels despite LDL-C control, consideration should be given to using an anti-inflammatory medication. While no single medication has been proven superior, considering previous studies, experience, affordability, and recent FDA approval97, colchicine appears to be the best available option. Therefore, we join in recommending hs-CRP as the biomarker of choice and colchicine as the preferred drug98. However, several questions need to be addressed to establish this protocol, such as the timing and frequency of hs-CRP testing after reaching target LDL-C levels, management of patients with renal failure or gastrointestinal intolerance, duration of colchicine prescription, and follow-up strategies after treating RIR. Furthermore, the suggested protocol does not address the potential treatment of patients with both low hs-CRP and LDL-C levels.

Figure 1. Mechanism of inflammation in atherosclerosis. ang II: angiotensin II; CD8: cluster of differentiation 8; ET-1: endothelin-1; ICAM-1: intercellular adhesion molecule-1; IFN: interferon; IL: interleukin; LDL: low-density lipoprotein; NLRP: nod-like receptor family pyrin; NO: nitric oxide; PDGF: platelet-derived growth factor; TNF: tumour necrosis factor; VCAM-1: vascular cell adhesion molecule-1; VEGF: vascular endothelial growth factor
Future perspectives
The future of atherosclerosis and RIR treatment lies in personalised medicine and novel medications. Ongoing research is focused on promising anti-inflammatory strategies and exploring new possibilities for atherosclerosis treatment99. One intriguing avenue is targeting efferocytosis, a process in which macrophages clear apoptotic tissue from plaques. Dysregulation of this process leads to increased inflammation and atherosclerosis. Additionally, advancements in nanotechnology offer potential breakthroughs by improving drug delivery, reducing systemic toxicity, and enhancing circulation time for drugs, including water-insoluble ones. In vitro and animal studies have already evaluated anti-atherosclerotic nanotherapeutic approaches. Personalised medicine represents a therapeutic approach that takes into account an individual’s medical history, environmental factors, genetic background, and molecular defects. A particularly promising avenue lies in mRNA-based therapeutics, which enables precise targeting of specific genes and holds great potential for the future of medical treatment.
Two crucial advancements worth considering to enhance established risk assessment methods are the use of updated models that incorporate the latest imaging technologies and the integration of artificial intelligence algorithms. In future trials related to colchicine in ACS, there will be a heightened focus on incorporating optical coherence tomography imaging guidance for secondary endpoints, as well as leveraging technologies such as cardiac magnetic resonance imaging, nuclear medicine, and sodium fluoride positron emission tomography/computed tomography100101. Furthermore, it is essential to underscore the importance of integrating hs-CRP into both existing and new cardiac risk algorithms.
Several key clinical trials are currently underway (Table 4) to examine the effects of different medications on RIR. For example, the ZEUS trial aims to evaluate the impact of ziltivekimab on reducing cardiovascular events in individuals with established ASCVD, chronic kidney disease, and inflammation102. The CONVINCE trial focuses on evaluating the use of colchicine in adults who have suffered an ischaemic stroke or transient ischaemic attack103. The CLEAR SYNERGY trial investigates the efficacy and safety of colchicine and spironolactone in patients with MI who underwent primary PCI104. Lastly, the GOLDILOX-TIMI 69 trial is a phase IIB study evaluating the anti-inflammatory potential of MEDI6570, a monoclonal antibody, and its effect on atherosclerotic and heart failure events in patients with a history of MI105. These clinical trials represent important efforts in advancing our understanding and treatment of atherosclerosis and RIR.
Table 4. Ongoing trials.
| Name of study | Aim |
|---|---|
| ZEUS 102 | Evaluating the impact of ziltivekimab on reducing cardiovascular events in individuals with established ASCVD |
| CONVINCE 103 | Evaluating the use of colchicine in adults who have suffered an ischaemic stroke or transient ischaemic attack |
| CLEAR SYNERGY 104 | Investigating the efficacy and safety of colchicine and spironolactone in patients with MI who underwent primary percutaneous coronary intervention |
| GOLDILOX-TIMI 69 105 | Phase IIB study evaluating the anti-inflammatory potential of MEDI6570, a monoclonal antibody, and its effect on atherosclerotic and heart failure events in patients with a history of MI |
| ASCVD: atherosclerotic cardiovascular disease; MI: myocardial infarction | |
Conclusions
Active primary prevention of RIR in atherosclerosis is crucial, as shown by extensive data. The convergence of biological evidence highlighting inflammation in atherosclerosis, the availability of biomarkers, and the effectiveness of anti-inflammatory medications demonstrated in clinical trials all call for their integration into future guidelines. We contend that the current evidence is substantial enough to advocate for the use of hs-CRP to assess patients with established atherosclerosis and the addition of colchicine to their medication regimen as a preventive measure, rather than waiting for a recurrent event to occur (Figure 2).
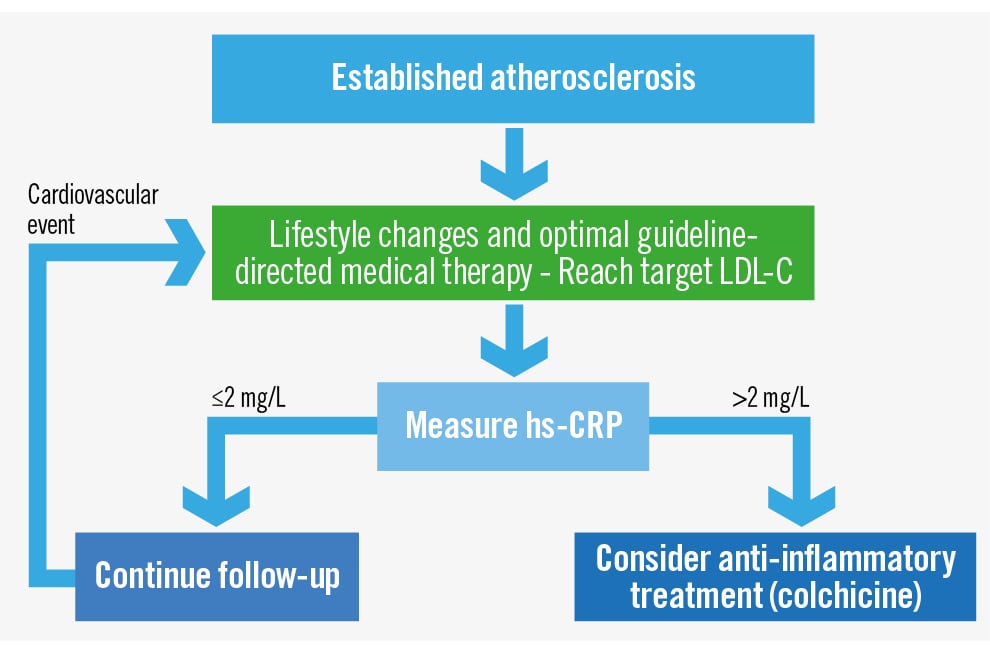
Figure 2. Suggested strategy for treating residual inflammatory risk. hs-CRP: high sensitivity C-reactive protein; LDL-C: low-density lipoprotein cholesterol
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
R. Waksman reports serving on the advisory boards of Abbott, Boston Scientific, Medtronic, Philips IGT, and Pi-Cardia Ltd.; being a consultant for Abbott Vascular, Biotronik, Boston Scientific, Cordis, Medtronic, Philips IGT, Pi-Cardia Ltd., Swiss Interventional Systems/SIS Medical AG, Transmural Systems Inc., and Venus MedTech; receiving institutional grant support from Amgen, Biotronik, Boston Scientific, Chiesi, Medtronic, and Philips IGT; and being an investor in MedAlliance and Transmural Systems Inc. I. Porto reports consultant or speaker fees from SysMedical, Medtronic, Abbott, Edwards Lifesciences, Abiomed, Terumo, Philips, Sanofi, Amgen, Daiichi Sankyo, AstraZeneca, Bayer, and Pfizer; and a department grant (minor) from Chiesi, Bayer, Medtronic, and Abbott. The other authors have no conflicts of interest to declare.

