Dear colleagues,
We have often insisted that our work advances by a healthy interchange between ourselves as clinicians and the industry that produces our devices. While our commitment to patients is foremost, few would deny that the quality of care, and the relentless evolution in what we offer our patients, is in part due to the vision of those who produce the tools we use –and help design. In earlier editorials I spoke about the technologies of the future, and how we apply them to the present (such as our infatuation –and use– of the iPAD), but here I want to speak about the past and how it impacts on the present: Cordis, one of the giants in the history of interventional cardiology is receding from the scene, and the fact has been little noticed by our community.
It is dramatic to see a pioneer in the field of drug-eluting stents (DES) disappear. DES is still 60%, 70%, even 80% of our practice, and as I look back at my career, I see all these great industry pioneers disappearing, one after the other. When I began my work, one of the leaders, the manufacturer of Andreas Gruentzig’s balloon was Schneider, and now Schneider is gone. Then in the US, there was U.S. Catheter & Instrument Corporation (USCI) / C.R.Bard…where are they now?
And what do we know about Cordis today? In 1994, the department of interventional cardiology at Johnson & Johnson was investing heavily, trying to introduce the Palmaz-Schatz stent, when after multiple failures and on the verge of stopping their endeavour we completed as an investigator designed study, the Benestent Trial that became with the USA Stress trial the scientific foundation for the approval of the US Regulator, the FDA. I was personally present at the FDA audition, with Martin Leon, Donald Baim and Sheldon Goldberg.
After that the momentum was increasing for bare metal stents (BMS), and J&J acquired Cordis in 1996.
As we know, it was a time of tremendous growth in interventional cardiology, for us, and for industry as well. With the financial and clinical success came many competitors such as the AV stent. Cordis developed the Crown Stent, which was not a success because of its
rigidity, and then produced the CrossFlex, which was good for bends, but not very strong. Then they took on-board the great PhD, Robert Falotico, developing the first DES, and in July 1999 I was in Warren, New Jersey when we completed the design of the very first FIM.
We all know the story of the RAVEL study and its results which seemed to eliminate the restenosis rate, and afterwards the great success, and, as in all success, people turning towards the leaders in the field, focussing a harsh spotlight on their technologies, trying to find their weaknesses. Cordis became the target of criticism –sometimes valid, often mixed with jealousy– but all-in-all they did pretty well. That was, until September 2006, when the famous ESC storm rocked our field, in some way targeting the main players, Cordis and Boston Scientific directly… remember, neither Medtronic nor Abbott had taken their current places as leaders, yet. There was a massive drop on both sides of the Atlantic in the use of the DES, with layoffs throughout the world, including here in The Netherlands.
Cordis addressed these challenges in a courageous way, continuing research and development and introducing such promising technologies as the Conor Stent. The Conor Stent was innovative, answering a real demand on the part of interventional cardiologists by offering us the possibility of multiple eluting reservoirs. We worked on a preclinical animal model with insulin; we wanted to test whether this specific stent could be used for release of insulin down into the infarcted area for the prevention of myocardial injury post infarction; allowing the glucose to enter the cell directly in the region of the myocardial infarction allowed us to do this. With the Conor Stent we saw the possibility of putting vascular endothelial growth factor (VEGF) in the reservoirs. The idea behind VEGF in total occlusions that could not be crossed was to put it in the stump of the total occlusion with bridging collaterals… the stent would deliver VGEF and increase the bridging collateral. There was another option, to put an analogue of adenosine, to have an increase in flow; in the acute phase of myocardial infarction flushing whatever was there, whether platelets, leucocytes, etc. However, through mechanical failures, the question of retention of the device on the balloon –something that is typically an engineering problem- the whole investment of the company in drug-eluting stents seemed to disappear.
In terms of innovation, I personally saw with my own eyes, a Conor biodegradable scaffold –they did it for me… they prepared the pattern, cut it in a polylactide– so that we could have the possibility of reservoirs on top of something that could itself disappear (Figure 1)… This is a dream that probably will become true one day.
Whenever a large corporation, with its complex and established upper management, has a wonderful product, the first thing they should not do is to capitalise on that product alone, but start on the next step in its evolution. In many ways Cordis attempted those next steps in evolving their products. We can never rest in a field that innovates daily, whether it is a stent or a technique, we must all move forward. This is a wake-up call for other industry partners who believe that they have the “ultimate” solution.
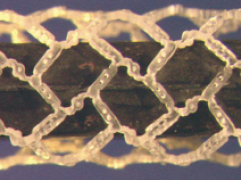
Figure 1. The Cordis sirolimus-eluting BRS (Warren, NJ, USA) with a common configuration of Conor Stents is designed to be fully absorbed within 18 months and is currently undergoing preclinical safety and biocompatibility studies. The sirolimus is incorporated within the polymer matrix at a dose, which is ten times higher than that found in the standard Cypher® stent, and elutes over a longer period. With kind permission of Robert Falotico.
Yet now, today, the real paradox is that when we study recent literature registries or the most recent randomised comparisons –despite all the negative things that have been shown in the past– they show excellent results for the Cypher Stent1-5. The legacy of Cordis has been, and thus continues to be, a vital one for our field, and so it is with sadness, but also with recognition and gratefulness, that we witness this pioneering giant disappearing from the scene.
References

