Abstract
Background: Fractional flow reserve (FFR) is correlated with angiographic and intravascular ultrasound assessments of stent placement. Post-stenting FFR has been described as a good predictor of clinical events after 6 months.
Objective: To evaluate the feasibility and clinical impact of targeting an FFR > 0.95 via incremental in-stent inflation pressures.
Methods: In this multicenter prospective study, 100 consecutive patients underwent FFR measurement at baseline, after balloon predilatation, and after stenting with 4-atm inflation pressure increments from 8 to 20 atmospheres. Inflations were stopped when FFR increased above 0.95 and angiographic stenosis was less than 20%.
Results: FFR > 0.95 was achieved in 81% of cases; this FFR target was reached at 8 atm in 47% of patients, 12 atm in 16 %, 16 atm in 15%, and 20 atm in 3%. Fifty percent of patients with adequate angiographic result had an FFR less than 0.95 and needed further higher inflations. FFR was correlated with residual stenosis when the total procedure was evaluated, and this correlation persisted when in-stent inflations alone were considered. Final FFR was significantly correlated with anginal status after 6 months.
Conclusions: Angiography guided PCI does not allow optimization of FFR. Since optimal post stenting FFR is correlated to better anginal status at 6-months, this suggests that FFR guided PCI is required to achieve optimal functional results of PCI.
ABBREVIATIONS
ANOVA: analysis of variance
ATM: atmosphere(s)
FFR: fractional flow reserve
IVUS: intravascular ultrasound
MACE: major adverse cardiac event
MLD: minimal luminal diameter
PCI: percutaneous intervention
QCA: quantitative coronary angiography
TIMI: thrombolysis in myocardial infarction
Intravascular ultrasound (IVUS) studies have established that inadequate stent deployment is common despite a satisfactory angiographic result and is associated with subacute thrombosis and restenosis1. This indicates both limited reliability of angiography for assessing stent deployment and a need for methods capable of optimizing stenting. Although drug-eluting stents are associated with decreased restenosis rates, optimal stent deployment remains crucial, most notably to avoid thrombosis2. High inflation pressures are often used in everyday practice to achieve maximum stent expansion. However, high pressures may damage both the vessel and the stent. The use of physiological variables to guide percutaneous coronary interventions (PCIs) has emerged in recent years. Physiological variables can be monitored during stenting via a 0.014 inch Doppler or pressure guide-wire3,4. As compared to IVUS, a pressure guide-wire is a simple and inexpensive tool for investigating conductance vessels, and a good correlation has been evidenced between the quality of stent implantation as evaluated by IVUS and post-procedural fractional flow reserve (FFR)5,6. FFR has shown promise as a tool for everyday clinical practice. Thus, in a large prospective multicenter registry study, better FFR values immediately after stenting were associated with better 6-month clinical outcomes, and the FFR cutoff being 0.957. However, FFR was not used to guide stenting, and FFR values > 0.95 were achieved in only 36% of patients. Another study using gradual balloon inflation pressures showed that FFR <0.96 predicted incomplete stent expansion by IVUS; this study also established that appropriate intracoronary adenosine doses were crucial to reliable FFR measurements5.
The objective of this multicenter study was to investigate a strategy of incremental balloon inflations up to 20 atmospheres targeting an FFR > 0.95, in terms of target achievement rates and of immediate and 6-month clinical outcomes.
Methods
Study population
The registry enrolled 100 patients managed in 10 French hospitals from April 2000 to June 2001. Patients were enrolled if they needed dilation and stenting of a single, de novo, native coronary stenosis measuring less than 15 mm in length and more than 2.5 mm in diameter. Patients with total chronic occlusion, marked calcified lesions, or type C lesions were excluded, as were patients with a history of myocardial infarction in the distribution of the target artery and those with multivessel coronary disease. A pressure-wire was used routinely as the angioplasty guide-wire (WaveMap, Jomed Inc, now Volcano Therapeutics Inc). Medical treatment during and after the procedure was according to the usual protocol in each participating center. All patients received aspirin (100 mg) in combination with ticlopidine (75 mg bid) or clopidogrel (75 mg daily) with an initial 300 mg loading dose, for at a minimum of 1 month. Written informed consent was obtained from each patient prior to the study. The institutional review board of the Henri Mondor Teaching Hospital approved the study for all the participating centers.
Angioplasty procedure and fractional flow reserve measurements
After injection of 5000 units heparin, predilatation balloon angioplasty at nominal pressure was performed. The balloon/artery and stent/artery ratio had to be 1.1 at nominal pressure (visual assessment). A 16 mm-long stent (Jostent Flex, Jomed) was used and expanded at 8 atm (nominal pressure). Direct stenting was not allowed to eliminate lesions which could not be fully dilated. FFR was measured using conventional methods after intracoronary injection of a 36-µg adenosine bolus (Krenosin™, Sanofi)8. Intraprocedural measurements were obtained at baseline, after balloon dilatation, and after each balloon inflation in the stent. After the 8-atm inflation, further inflations were performed at 4-atm increments (12, 16, and 20 atm) as needed to achieve FFR >0.95 and residual stent stenosis by quantitative coronary angiography < 20 % (figure 1).
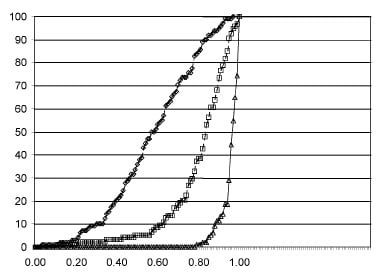
Fig. 1. In-hospital and 6-month clinical follow-up
Follow-up
Clinical data were obtained at hospital discharge and 6 months after the procedure. Troponin T was assayed in all patients 24 hours after the end of the procedure (normal, < 0.5 (g/l). Major adverse cardiac events (MACE) were defined as death due to a cardiovascular cause, non-fatal acute myocardial infarction, repeat target vessel revascularization by angioplasty or surgical coronary artery bypass, and any procedure related complication requiring a major intervention or prolonged hospital stay. All patients underwent a standard treadmill exercise test and were classified according to the Canadian Cardiovascular Society classification system for angina pectoris.
Quantitative coronary angiography
Online quantitative coronary angiography was performed before the procedure then after balloon predilatation and after each in-stent inflation. All patients were adminstered a vaso-dilating drug before and at the end of the procedure (intracoronary molsidomine or nitroglycerin). Off line QCA of two orthogonal views were performed by an independent investigator who used the CAAS II system, Pie Medical (AP-HP Core Lab, Henri Mondor Teaching Hospital, Créteil France). A 6F guiding catheter, free of contrast, was used for calibration. The minimal luminal diameter (MLD) and reference diameter proximal to the lesion were measured as previously described9. The inter- and intra-observer variations for QCA diameter stenosis were 9 ± 12 % and 7 ± 14 %, respectively.
Statistical analysis
Continuous variables are expressed as a mean standard deviation. ANOVA was used to compare QCA and FFR data obtained at baseline, after the inflations, and after 6 months. When statistically significant within subject variations were observed, two-tailed paired t-tests (or Wilcoxon tests for nonparametric data) were used. Differences in continuous variables were evaluated using a two-tailed unpaired Student’s t-test (or a Mann-Whitney test for nonparametric data). Statistical correlations for angiographic and FFR parameters were calculated using forward stepwise regression analysis. Chi-square tests were used to compare categorical variables. P values 0.05 were considered statistically significant.
≥
Results
Patient population and dilatation procedure
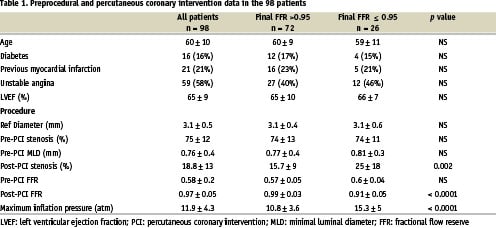
Patient characteristics are shown in table 1. Mean age was 60 years (38 to 78 years) and 82.6 % of patients were men. Procedural success defined as a residual stenosis < 20% and TIMI 3 flow was achieved in 99 % of patients. In 1 patient, FFR was normal after balloon angioplasty and, consequently, stenting was not performed (protocol violation); another patient had a stenting failure with acute coronary occlusion and myocardial infarction. Both these patients were removed from the data analysis. In 10 patients, dissection required use of more than 1 stent (two stents in 9 patients and three stents in 1 patient); dissection occurred after balloon predilatation, i.e., was not induced by in-stent inflation. The ratio of mean balloon and stent nominal diameter over mean reference artery diameter was 1.06. In the overall population, baseline FFR was 0.58 ± 0.2, reference artery diameter was 3.09 ± 0.47 mm, minimal lumen diameter (MLD) was 0.76 ± 0.37 mm and stenosis was 75 ± 13%.
Results of the angioplasty procedure
In all, 190 in-stent balloon inflations were performed, and FFR values greater than 0.95 were achieved in 81% of patients. As shown in figure 2, mean FFR increased to 0.81 ± 0.16 after balloon predilatation and to 0.97 ( 0.05 at the end of the procedure (P < 0.0001). The inflation pressure needed to reach the target FFR value (> 0.95) was 8 atm in 47% of cases, 12 atm in 16% of cases, 16 atm in 15% of cases, and 20 atm in 3% of cases. Of all normalised final FFR, 57% was obtained at 8 atm, 21% at 12 atm, 19% at 16 atm and 3% at 20 atm. As expected, the inflation pressure used last was significantly higher in the patients who failed to reach the FFR target than in the other patients (15.3 ± 4.9 vs. 10.8 ± 3.5; P < 0.0001).
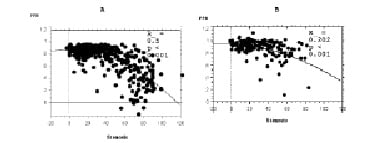
Fig. 2. Distribution of FFR values at baseline, after balloon dilatation, and after stenting.
Stenosis as assessed by QCA decreased to 45 ± 16% after balloon predilatation and to 18 ± 12% at the end of the procedure (P < 0.0001). Residual stenosis decreased with increasing in-stent inflation pressures up to 16 atm (21 ± 14% after 8 atm, 16 ± 8 after 12 atm, and 12 ± 11 after 16 atm) but showed no further decrease after inflation at 20 atm (18 ± 13%). These results were not explained by different stent / artery diameter ratios.
Agreement between fractional flow reserve values and angiographic stenosis
Both the target FFR and QCA stenosis < 20% were reached in 77.5% of the population overall and in 47% of cases after 8 atm, 20% after 12 atm, 21% after 16 atm, and 12% after 20 atm. After inflation to 8 atm, among 71 patients who had stenosis < 20 %, only 34 (52%) also had FFR > 0.95; corresponding proportions were 26 (51%) of 51 patients after 12 atm and 19 (38%) of 29 patients after 16 atm. This entails that 52% of inflations were done on the basis of insufficient FFR despite satisfactory angiographic result.
When we pooled all the data collected at the various steps, we found a significant second-degree polynomial relation between lesion stenosis by QCA and FFR (r = 0.71, P < 0.001). This relation was present but weaker when each inflation step was considered separately (r = 0.45, P < 0.001) (figure 3).
Figure 3: When all data obtained for all patients at the different steps of the procedure were pooled, FFR was significantly correlated with stenosis (A) Interestingly, this correlation persisted when we focused on in-stent inflation pressures (B).
Follow-up
No clinical events were recorded during the hospital stay. Cardiac troponin levels increased in 28 patients, although the levels consistently remained less than 3-fold the baseline value. Troponin elevation was not correlated with the number of stents, residual stenosis, number of balloon inflations, or final inflation pressure. Troponin levels 24 hours after the procedure were not significantly different between patients with optimal versus suboptimal final QCA and FFR results (0.8 ± 1.6 vs. 1 ± 1.4 (g, respectively).
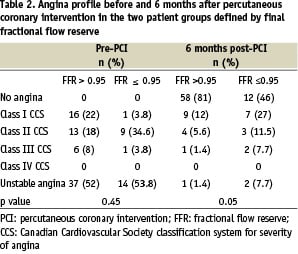
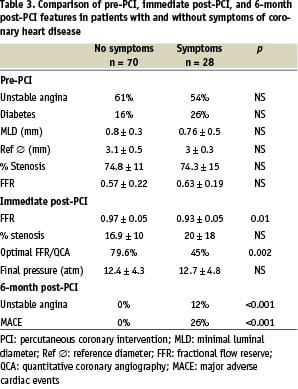
Data were available for all patients 6 months after the procedure. No cases of death or myocardial infarction were recorded. About 70% of the patients were symptom-free, 26% had stable angina, and 3% had unstable angina (Table 2). Final FFR was significantly lower in patients with than without angina after 6 months (0.94 ± 0.03 vs. 0.97 ± 0.05; P = 0.04) (Table 3). Patients whose FFR and QCA goals were reached after the procedure were more likely to be free of angina after 6 months than were the other patients (19 vs 52%, P = 0.006). The treadmill exercise test was positive in 17 (19%) patients, and this was related to the final in-stent inflation pressure (P = 0.02) but not to final FFR or to final stenosis by QCA.
Eight patients (8%) experienced MACE as defined in the methods chapter. Three of these patients had unstable angina. All 8 patients underwent a repeat revascularization procedure consisting in repeat angioplasty (n = 5) or bypass surgery (n = 3). Neither final FFR nor residual stenosis by QCA predicted MACE occurrence.
Discussion
The present study shows that stepwise FFR measurements are useful to guide stenting in everyday practice. In this multicenter study, the FFR target (> 0.95) was achieved in approximately four fifths of patients. Although inflation to 16 atm was often used, the highest pressure of 20 atm was rarely required and should be limited. This target FFR-guided strategy was not associated with an increase in adverse events. In addition, improved 6-month clinical outcomes were noted in patients whose target FFR was achieved and whose QCA stenosis was < 20 % immediately after the procedure.
Optimal stent expansion
In previous studies, QCA systematically overestimated luminal gain or final MLD achieved by PCI, as compared to IVUS thereby resulting in overoptimistic estimates of results10,11. IVUS showed a high rate of inadequate stent expansion despite apparently satisfactory angiographic results and a potential need for high pressures to achieve full stent deployment, although even this was not consistently successful12,13. However, high pressures may carry a risk of arterial wall damage and increased rates of late restenosis14. Recent studies found that FFR was cheap and as effective as IVUS for monitoring stent deployment6. FFR values lower than 0.96 after stenting were associated with suboptimal IVUS angioplasty results. Conversely, a post-stenting FFR greater or equal to 0.96 does not always indicate optimal stent expansion5. Beside this imaging discussion, a cut off FFR value of 0.95 provided the best clinical performance when adequate intracoronary adenosine is administered7.
These conclusions suggest that a strategy for reaching FFR > 0.95 should be developed. A multicenter registry study of everyday clinical practice without stratification on stenting strategies showed that 50% of patients had FFR values below the target yet had acceptable QCA results7. This is consistent with the results of our study which show that at each step of inflation pressure half of the patients had low FFR despite residual stenosis ≤20%. This emphasises the role of FFR in stenting strategy. However, in the population with high post-stent FFR values, clinical outcomes were good, with few cardiac events. In the FFR-targeted strategy, inflation pressures are increased until the FFR value is greater than 0.95, as measured after administration of an adequate adenosine dose. The objective of the present study was to evaluate this strategy. We found that the target FFR could be reached in more than four fifths of patients. Interestingly, the target FFR was achieved in 60% of cases with inflation pressures no greater than 12 atm, i.e., within the range used routinely. However, higher pressures were needed in more than 20% of the patients. Among patients whose FFR was below the target after 16 atm, few reached the target after 20 atm. The complementary utility of coupling both FFR and angiography measurements was evidenced by the fact that 20 atmospheres inflation pressures were still used in 3% of the patients to obtain both satisfactory angiographic and FFR results. In 77.5% of the overall population, both the target FFR and desired angiographic results were obtained. This proportion was only 60% in a study by Hanekamp et al. in which the target FFR was 0.946.
Failure to normalise FFR for all patients
In the present study, failure to reach the FFR target despite incremental inflation pressures up to 20 atm was noted in 19% of patients, in keeping with results obtained in an IVUS study by Schiele15. Thus, whether inflation pressures are determined based on a physiological variable (FFR) or a geometric variable (measured by IVUS), about 20% to 30% of lesions seem to resist satisfactory correction by angioplasty6. In our study, at 20 atmospheres, in contrast to lower pressures, low FFR is related to residual angiographic stenosis probably determined by abnormal arterial wall structure. Thus, inflation pressures of 20 atm are unlikely to provide additional lesion correction as compared to lower pressures. The same finding was described by Hannekamp et al who could not normalise FFR with pressure above 12 atm in old generation Wiktor stents16. In an IVUS study, the effect of balloon or stent dilatation was influenced by the presence and amount of calcifications17. In the present study, inflations with larger balloons rather than higher pressures might have improved FFR. However, the final balloon/artery ratio was close to 1.2, and optimal stent expansion has been shown to occur with ratios of 1.1 or more. In addition, distal micro-embolism or other mechanisms altering the microvascular bed and causing diffuse chronic impairment of the myocardial reserve cannot explain the failure to normalise the FFR, as decreased flow reserve would be expected to increase FFR values.
Six month outcomes
In a recent study in which FFR was measured but not used as a treatment target, only 35% of patients had FFR >0.96 after stent placement. Interestingly, the 6-month event rate was decreased by 50% in these patients as compared to those with lower FFR values7. Our study was not designed to compare various treatment strategies but instead was aimed at assessing the feasibility of using an FFR target to determine inflation pressures during stenting. Nevertheless, our finding that achieving the FFR target was closely associated with absence of angina after 6 months strongly suggests that FFR monitoring during stenting may improve clinical outcomes. We found no association between final FFR and MACE after 6 months, whereas in a multicenter registry study by Pijls et al. FFR was independently associated with MACE at the same timepoint7. This discrepancy may be ascribable in part to the smaller sample size in our study (100 vs. 750 patients); in addition, the very low event rate (9%) limited the statistical power of our study. Conceivably, the low MACE rate in our study may be ascribable to the fact that the vast majority of our patients (81%) achieved the FFR target.
Study limitations
Normal myocardial function was an inclusion criterion for our study. Even if it has been reported that a cut off FFR value of 0.75 indicates normal flow through arterial lesions in patients with ischemic cardiomyopathy13,15, coronary reserve is decreased in various non-ischemic and ischemic cardiomyopathies. Whether 0.95 would be an appropriate target FFR value for coronary stenting evaluation in patients with cardiomyopathy needs to be evaluated.
This study protocol did not allow for direct stenting, which is performed in 50% of coronary angioplasties in France. This was a feasibility study, and we wanted to eliminate a potential influence of undilatable calcified lesions on our results. Undilatable calcified lesions preclude full stent deployment, increasing the likelihood of suboptimal functional results. If patients with such lesions had been included, the FFR normalisation rate with a pressure of 8 atm may have been lower than the 47% obtained in our study.
The results from an in vitro study suggest that FFR measurement via a pressure wire may be unreliable for evaluating stent deployment18 and that QCA and IVUS may be more effective. However, this study has numerous limitations, as pointed out by the authors and by Naidu and Bailey in an editorial emphasizing that the value of FFR should be assessed on the basis of clinical data19. We found a significant correlation between FFR and stenosis when all the data from baseline to completion of angioplasty were pooled. This correlation has been reported previously, but in addition we showed that it persisted when we considered only the in-stent inflations. Although this correlation does not necessarily mean that FFR reflects the quality of stent placement, it does indicate that FFR reflects the geometric effects of stent placement. Furthermore, it is consistent with the previously reported correlation between FFR and strut apposition.
Conclusions
Final geometry after stenting is a strong predictor of adverse clinical outcomes. Several studies have established that physiological or ultrasound variables can serve to optimize immediate post-procedural results. However, these strategies did not consistently improve clinical outcomes and re-stenosis rates, although none had deleterious effects. Lack of statistical power complicates the interpretation of many of these studies. Larger randomised studies are needed to validate the target-variable strategy for stenting, particularly as the results obtained with the recently introduced coated stents seem highly dependent on the quality of stent placement20,21.
Acknowledgment
FROST Study Group
Participating Hospitals and Investigators: Hôpital Henri-Mondor, Créteil (J.L. Dubois-Randé, P. Dupouy, A. Belarbi, E. Teiger, F. Veyssière), Hôpital La Cavalle Blanche, Brest (J. Boschat, M. Gilard), Hôpital d’Angers (A. Furber), Clinique Saint-Martin, Caen (P. Commeau, B. Huret, J.F. Morelle), Hôpital Privé d’Antony, Antony (E. Aptecar, J.M. Pernès, N Bussy), Hôpital Antoine Béclère, Paris (M. Slama, I. Carel), CH de Bretagne Sud, Lorient (P. Cazaux), Hôpital Bichat, Paris (L.J. Feldman, P.G. Steg), Hôpital Georges Pompidou, Paris (A. Lafont), Hôpital Necker, Paris (J.P. Metzger), Hôpital Privé Les Franciscaines, Nîmes (O. Wittemberg)
Excerpt from the reviewers
The study does actually not allow to determine whether a strategy described in the study is better than another one in which one would inflate all the stents with a pressure of 16 atm. To answer this (purely academic) question a different design is needed. The most logical conclusion of the present study might probably be summarised as follows: “It is better to deploy all stents at 16 or 20 atm. This provides an FFR>0.95 in most patients. Angiographically the result looks good at 8 atm but this result is not confirmed when FFR is measured”. In addition, the study confirms that patients with a final post stent FFR>0.95 as measured in the distal part of the vessel have a better clinical outcome. This is not truly novel as a registry encompassing 7 times more patients was published earlier (reference 7).

