Abstract
Aims: To investigate the medium term (2 year) clinical outcome of the use of the paclitaxel-eluting stent (PES) compared to the sirolimus-eluting stent (SES). To date, there are no direct comparative data on the efficacy of these stents over medium term follow-up. Furthermore, a possible late restenotic phenomenon has not been excluded.
Methods and results: The Taxus-Stent Evaluated At Rotterdam Cardiology Hospital (T-SEARCH) registry compared 576 consecutive “all-comer” patients, exclusively treated with PES, with 508 patients who received SES from the RESEARCH registry in the preceding period. Patients were enrolled irrespective of clinical or angiographic features. At 2 years, major adverse cardiac event (death, myocardial infarction or target vessel revascularisation) rates were comparable in the two groups: 15.4% in the SES group versus 18.9% in the PES group (HR 1.26, 95% CI 0.94-1.69, p=0.12). Correcting for differences in both groups resulted in an adjusted HR of 1.11 (95% CI 0.82-1.50, p=0.51, using significant univariate variables). Target vessel revascularisation was 8.0% in the SES group compared with 9.6% in the PES group (HR 1.23, 95% CI 0.81-1.86, p=0.33).
Conclusions: The unrestricted use of SES and PES was safe at two years of follow-up. No significant difference was found between the two devices in terms of death or MI, MACE, TVR or TLR. No late clinical restenotic phenomenon was observed.
Introduction
Several years have elapsed since the commercial introduction of sirolimus and paclitaxel eluting stents and to date more than 3 million drug-eluting stents have been implanted worldwide.
Both stents, loaded with antiproliferative drugs, have shown to be highly effective in reducing restenosis in stenotic coronary arteries compared to bare metal stents1-5. Evidence was seen from the early days of DES with trials describing the effect of the Rapamycin covered SES in relatively simple lesions in the FIM trial6,7 and the RAVEL trial8. Currently, randomised trials, such as the REALITY, TAXI, CORPAL, SIRTAX, ISAR Diabetes trial and BASKET trial, are comparing SES and PES in a wide variety of patient and lesion types9-14. Although a recent meta-analysis has demonstrated the efficacy of DES, and the superiority of SES to PES in reducing restenosis and target lesion revascularisation (TLR), the randomised REALITY trial did not show a difference in binary restenosis and neither in major adverse cardiac events (MACE) at 8 and 12 months between both devices9,15. Of note, no difference was seen in the rates of death and myocardial infarction after relatively short-term follow-up in both studies.
The purpose of the present study is to report the two-year clinical outcome of an unselected patient cohort compromising 1,084 consecutive patients treated with a sirolimus- or paclitaxel-eluting stent. The study was performed for two reasons: First, to evaluate the incidence of late adverse events – the importance of this issue can be demonstrated by a variety of complications that showed up after several years of clinical experience using multiple drug-eluting devices such as; stent thrombosis more than one year after implantation despite continuation of anti-platelet therapy16, delayed neointimal growth17, and a phenomenon of late restenosis in porcine models18. Secondly, to see whether both devices were still associated with a comparable outcome at 2 years19.
Methods
Study design and patient population
The Taxus-Stent Evaluated At Rotterdam Cardiology Hospital (T-SEARCH) registry is a single-centre prospective registry with the aim of evaluating the safety and efficacy of the unrestricted use of PES (PES, TAXUS, Boston Scientific Corp., Natick, Massachusetts, USA) implantation in an unselected patient population typical of daily practice. Its design and methodology are similar to that of the RESEARCH registry20 and follows the dynamic registry design described by Rothman and Greenland21.
PES was granted Conformité Européenne (CE) approval on February 16, 2003 and replaced SES (SES, Cypher, Cordis corporation, Warren, NJ, USA) as the default strategy for every percutaneous coronary intervention (PCI) in our institution. Up to September 30, 2003, a total of 576 patients with de novo lesions were treated exclusively with PES and are included in the present report (PES group). In this period, 84% of all patients with de novo disease received a PES. Patients not treated purely with PES or patients included in other drug-eluting stent trials were excluded from the present report. This PES group was compared with a control group that comprised the active arm of the RESEARCH registry, including 508 patients with de novo disease treated solely with SES (SES group). Written informed consent was acquired for every patient included. Our study fulfilled the criteria of the declaration of Helsinki and was approved by the hospital ethics committee.
Procedures and post-intervention medications
All procedures were performed according to current standards, with the final interventional strategy (including direct stenting, postdilatation and the use of intravascular ultrasound) left to the operator’s discretion20. Angiographic success was defined as residual stenosis <30% by visual analysis in the presence of Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow. All patients were pretreated with 300mg clopidogrel. For patients in the SES group post procedure clopidogrel (75 mg/day) was prescribed for at least 3 months. Several exceptions were made for patients treated for long lesions (stented segment >36 mm), chronic total occlusions, bifurcations and patients in whom more that 3 stents were used. These patients received at least 6 months clopidogrel. In the PES population clopidogrel (75 mg/day) was prescribed for at least 6 months according to the protocol of several randomised clinical trials3,5. Furthermore, all patients were advised to maintain life-long aspirin treatment (at least 80 mg/day).
End point definitions
Our primary endpoint was MACE at 2 years. MACE was defined as a composite of all cause death, non-fatal myocardial infarction (MI) or target vessel revascularisation (TVR). Secondary endpoints were target lesion revascularisation (TLR), defined as a treatment of a lesion in-stent or within 5 mm of the stent borders, and clinically driven repeat revascularisation, defined as any intervention motivated by a significant luminal stenosis (>50% diameter stenosis) in the presence of anginal symptoms and/or proven myocardial ischaemia on the target vessel territory by noninvasive testing. Myocardial infarction was diagnosed by a rise in creatine kinase-MB fraction (CK-MB) of three times the upper normal limit according to American Heart Association/American College of Cardiology guidelines22,23. Subacute angiographic stent thrombosis was defined as an angiographically documented complete occlusion (TIMI grade 0 or 1 flow) or a flow-limiting thrombus (TIMI grade 1 or 2 flow) in the first 30 days after a successful procedure. Late angiographic stent thrombosis was defined as late – occurring at least one month after DES implantation with acute symptoms; angiographic – stent thrombosis confirmed angiographically; stent thrombosis – defined as thrombosis with TIMI grade 0 or 1 flow or the presence of a flow limiting thrombus (TIMI flow 1 or 2)24.
Two-year follow-up data
Long-term survival status was obtained by information provided by the municipal civil registry. Subsequently, questionnaires were sent to all living patients inquiring about new interventions (either surgical or percutaneous), myocardial infarction and medication usage. If patients had an MI or underwent a re-intervention in another hospital, discharge letters from the referring hospitals were requested and analysed for additional information. In cases of doubt, the local cardiologist or general practitioners were contacted. All information was prospectively collected in a dedicated database. We were not able to retrieve complete follow-up information on 30 patients, mostly due to emigration or due to an illegal status in the Netherlands. Finally, follow-up was available for 97% of the patients in both groups.
In both groups, follow-up coronary angiography was clinically driven by symptoms or signs suggestive of myocardial ischaemia or mandated by the operator at the end of the index procedure predominantly for complex procedures. In the PES group 18.4% underwent angiographic follow-up, as part of three specific complex subgroups: left main stenting, crush-bifurcation procedures, and patients who were part of a vulnerable plaque substudy. Of the SES patients, 36.0% underwent angiographic follow-up, as part of the following complex subgroups: bifurcation lesions, chronic total occlusions, very small vessels, left main stenting, long stent length (36 mm), and acute MI.
Statistical analysis
Continuous variables are presented as mean ±SD and were compared by Student’s t-test. Categorical variables are presented as counts and percentages and compared by Fisher’s exact test. All statistical tests are 2-tailed and a p-value <0.05 was considered significant. The cumulative incidence of adverse events was estimated according to the Kaplan-Meier method, and Cox proportional hazards models were used to assess differences between the two strategies. Curves were compared by log-rank test. Separate Cox proportional hazards models were performed to identify independent predictors of adverse events, using clinical, angiographic, and procedural variables contained in Tables 1 and 2.
The Cox proportional hazards regression models were used to control for differences between groups, and the final results are presented as adjusted hazard ratios (HRs). Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored.
Results
Baseline and procedural characteristics
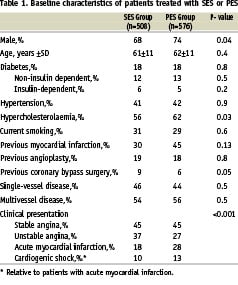
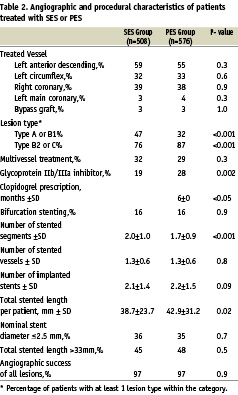
Both baseline and procedural characteristics are shown in Tables 1 and 2. In summary, patients were predominantly male and slightly more frequent in the PES group, PES patients had more myocardial infarctions (MIs) and cardiogenic shock as their presenting symptom and hypercholesterolaemia was more often present. Furthermore, PES patients had more complex lesions, received longer total stent lengths and glycoprotein IIb/IIIa inhibitors were administered more frequently (28% versus 19%; p=0.002). Furthermore, fewer PES patients had a history of previous coronary bypass surgery, and fewer segments per patients were stented, although the number of vessels treated per patient was identical. Other baseline and procedural characteristics were similar.
Two-year follow-up
The one-year results of our study have been published previously19. At two years of clinical follow-up there was no significant difference in mortality between the SES- and PES-groups, (5.8% versus 7.7% respectively, HR 1.35, 95% CI 0.84 - 2.16, p=0.21) (Figure 1a).
No difference was found in the combined endpoint of death or MI in the SES- versus PES-group (9.8% versus 11.9%, HR 1.23, 95% CI 0.85-1.78, p=0.26) (Figure 1b). TLR and TVR rates were similar in both groups. Cumulative incidence of TLR was 6.8% versus 6.3% in the SES group versus the PES group respectively (HR 0.94, 95% CI 0.58-1.51, p=0.79) and TVR was 8.0% in the SES-group versus 9.6% in the PES-group (HR 1.23, 95% CI 0.81-1.86, p=0.33) (Figure 1c). Clinically driven TVR was performed in 7.2% of the SES group compared with 9.4% in the PES group (HR 1.34, 95% CI 0.87-2.06, p=0.18). The two-year cumulative incidence of combined MACE was 15.4% in the SES-population versus 18.9% in the PES-population (unadjusted HR 1.26, 95% CI 0.94-1.69, p=0.12) (Figure 1d).
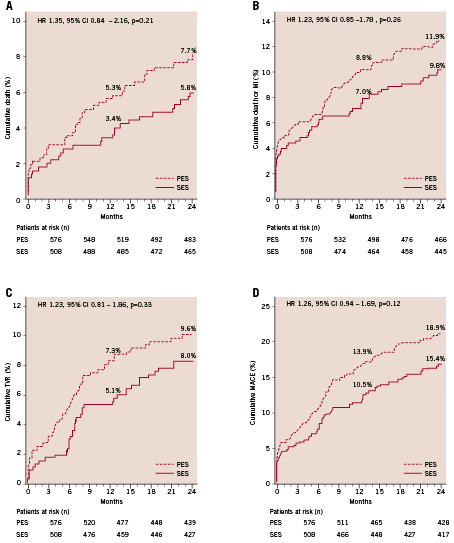
Figure 1. Two-year adverse events in patients treated with sirolimus- and paclitaxel-eluting stents (SES and PES). Cumulative risk of death (A); death or myocardial infarction (MI) (B); target vessel revascularisation (C); death, myocardial infarction or target vessel revascularisation (MACE) (D).
Late stent thrombosis at 2 years occurred in 0.3% of the PES group compared with 0.2% in the SES group. Total rate of stent thrombosis was 1.6% in the PES group versus 0.6% in the SES group (p=0.15).
Events between one and two years
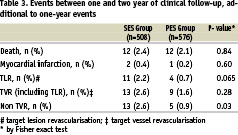
In this period a total of 48 events occurred. Twelve patients died in the SES group, two died of a cardiac cause, 6 patients died of a non-cardiac cause, 2 patients died suddenly of unknown cause and of 2 patients the cause of death was unknown. Twelve patients died also in the pre-SES group, 5 of a cardiac cause, 3 patients died of non-cardiac causes and the cause of death of 4 patients was unknown. Three MI’s occurred (two in the SES group versus one in the PES group). Furthermore, 13 patients received a TVR in the SES group versus 9 in the PES group, all of them were clinically driven. Eleven patients treated with SES underwent a target lesion revascularisation and only 4 out of the PES population (p=0.065). Additionally, 13 patients treated with SES and 5 with PES required a repeat intervention in a different vessel. One case of late-stent thrombosis was reported between one- and two-years.
Predictors of adverse events
In order to identify independent predictors of MACE at two years of follow-up, Cox regression analysis was performed for all baseline characteristics listed in Tables 1 and 2. The following variables were significant in predicting MACE at two years of follow-up: age > 65, female gender, diabetes mellitus, multivessel disease, left main stenting, bifurcation stenting, lesion type B2 or C and total stented length (per 10 mm increment) (Table 4).
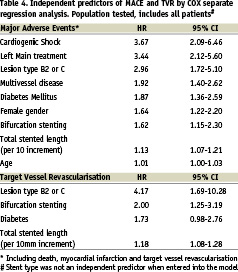
A second analysis was performed to determine independent predictors of TVR. Diabetes mellitus, lesion type B2 or C, bifurcation stenting and total stented length per 10 mm increment were found to be significant. Subanalyses were performed in several subgroups according to baseline and procedural characteristics (Figure 2).
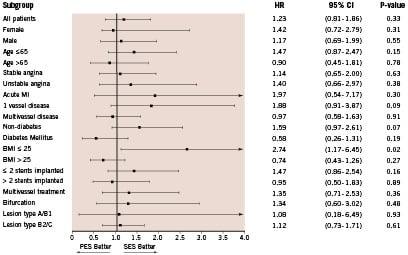
Figure 2. Hazard ratios (HR) of stent type at two-year follow-up for target vessel revascularisation in subgroups of patients according to baseline and procedural characteristics. MI=myocardial infarction.
In patients of normal weight, defined as body mass index (BMI) <25, patients treated with SES had a significantly lower rate of TVR compared to the PES group (p=0.02). In all other subgroups no superiority was noticed between SES and PES.
Adjustment for differences between both groups
The Cox proportional hazards regression model was used to adjust the two groups by correcting for multiple potential confounders in the baseline and procedural characteristics. First, a model was built forcing stent type and all independent predictors listed in Table 4. All previously significant variables remained significant except for bifurcation treatment, age and total stented length. The adjusted HR for use of PES became even less significant, decreasing from HR 1.26 (95% CI 0.94-1.69, p=0.12) to HR 1.12 (95% CI 0.83-1.51, p=0.44), after controlling for the increased complexity in the PES group.
A second model was then built forcing stent type and significant univariate variables (independent predictors plus number of stents), and the adjusted outcome of MACE at two year was similar between SES and PES (adjusted HR 1.11, 95% CI 0.82 to 1.50, p=0.51).
Finally, stent type was also not a significant predictor of TVR when adjusted for lesion type, bifurcation stenting, diabetes and total stented length (adjusted HR 1.09, 95% CI 0.72-1.66, p=0.68).
Discussion
The present study reports on the 2-year clinical outcome of the use of SES and PES in a real world patient cohort and confirms that neither one of both devices is superior to the other in preventing MACE. Additionally, no differences were found in the occurrence of death and MI, TLR and TVR between both groups.
Death and MI occurred in a similar amount in both groups, which is in accordance with randomised trials15. Additionally, there was a trend towards a higher incidence of stent thrombosis in the PES group. It has to be mentioned that in the second year, 2 patients in the SES group and 3 patients in the PES group died of sudden death of unknown cause or of a fatal out-of-hospital cardiac arrest. It cannot be excluded these patients also suffered late thrombotic events.
Whether the SES is superior to PES in terms of late luminal loss and the ability to reduce the need for re-interventions, is still a topic of debate. In terms of target lesion revascularisation, both the SIRTAX and ISAR-DESIRE showed TLR rates favouring SES12,25. However, the TAXI, REALITY, ISAR-DIABETES and CORPAL studies did not show a difference between both devices9,10,13,26. In terms of angiographic restenosis, both the SIRTAX and ISAR-DIABETES showed results favouring the SES. Although, it has to be mentioned that in the SIRTAX trial the incomplete angiographic follow-up may have resulted in an overestimation of the difference owing to attrition bias. However, the medium to long-term difference remains unknown. The present study reports the 2-year clinical follow-up of the use of the sirolimus- and paclitaxel- eluting stents in a real world patient cohort and confirms that neither one of the devices is superior to the other in preventing the need for revascularisations or the occurrence of overall MACE. The incidence of baseline characteristics with a predictive value towards a worse outcome was higher in the PES cohort and the lesions treated in the PES patients were more difficult overall. This is reflected in the adjusted MACE rate, in which the difference becomes even smaller. Additionally it has to be mentioned that between one and two years, the TLR rate is in favour of the paclitaxel-eluting stent (2.2% versus 0.69%; p=0.065).
When compared to the PES patients, a significantly higher amount of SES patients underwent angiographic follow-up. Of all patients with angiographic follow-up, only 6 (6.6%) underwent a TVR because of a significant (>50% stenosis) without “documented” anginal symptoms. Of note, 2 were because of severe proximal stenosis with large areas of myocardium at risk. Thereby, all TVRs performed in the second year were clinically driven. It is for this reason, that we did not choose clinically driven TVR as a primary endpoint.
An additional rebound phenomenon, as seen in porcine models and brachytherapy, does not seem to occur, at least after two years of follow-up. This latter is supported by the FIM study with 4 years of angiographic follow-up, which demonstrated the absence of a catch-up phenomenon of restenosis in a small patient population7.
Although both sirolimus- and paclitaxel-eluting stents have been shown to reduce neointimal proliferation, their mechanisms of action are different. Both devices modify the healing process after stent injury, which is the most likely explanation for the reduction in restenosis27. Nevertheless, both drugs interfere with a different part of the cell cycle and both stents have different polymer coatings and dissimilar drug-release kinetics28,29. The clinical implications in the differences between both devices still have to be determined.
Looking at the independent predictors of MACE (table 4), it can be concluded that patients treated for left main stenosis or complex lesions (type B2/C) had a significantly higher risk of adverse events at 2 years. Several trials, like the FREEDOM, COMBAT, SYNTAX and CARDIA trial, are currently ongoing to see whether these patients would benefit more from coronary artery bypass surgery30,31.
In the subgroup analyses, we found that patients with a BMI <25 treated with SES had a superior outcome with respect to the need for TVR when compared to PES. A paradox in the relationship between BMI and late mortality after PCI with bare-metal stents has been previously described, however, repeat revascularisation rates were not shown to be affected by body mass32. Whether the “obesity paradox” will extend to repeat revascularisations in the DES-era and will be influenced by the type of DES needs further investigation.
We realize this is an observational, non-randomised cohort study and thus suffers from its design. For instance, the two sequential cohorts are separated by a 4-month interval, resulting in several differences in both baseline and procedural characteristics. In general, the PES population was more complex overall. More primary PCIs were performed, because of the implementation of a pre-hospital protocol that triaged more patients to primary PCI. More complex (Type B2/C) lesions were treated and more stents were implanted. This latter is likely due to the growing confidence in the superior properties of DES compared to BMS.
However, our study comprises an all-inclusive unrestricted patient population which is able to represent the daily clinical practice of a large catheterisation laboratory and thus may possess a greater generalisability than has been possible with randomised trials.
Conclusion
The medium term follow-up of the T-SEARCH registry shows that the unrestricted use of SES and PES was still safe at two years. No significant difference was found in the adjusted outcome of both devices in terms of death or MI, TLR, TVR or MACE. The inferior trend in crude outcome seen in PES was, in part, due to its higher-risk population. A trend towards less TLR in the PES group between one and 2 years was also observed. No late clinical restenotic phenomenon was seen in either group and stent thrombosis after one year occurred in only one patient.
Acknowledgement
The following operators were involved in the procedures of the discussed patient population:
Chourmouzios A. Arampatzis, MD
Eugene McFadden, MD, PhD
Pim J. de Feyter, MD, PhD
Willem J. van der Giessen, MD, PhD
Sjoerd H. Hofma, MD, PhD
Angela Hoye, MBChB, MRCP
Peter P.T. de Jaegere, MD, PhD
Evelyn Regar, MD, PhD
Patrick W. Serruys, MD, PhD
Georgios Sianos, MD, PhD
Pieter C. Smits, MD, PhD

