Introduction
Aortic stenosis is the most common valvular disease in the elderly. Age, in addition to co-morbid conditions, is the reason why a large number of elderly patients are not referred or even rejected for surgical valve replacement.1 These patients may be candidates for percutaneous aortic valve replacement (PAVR). Suitability for this treatment mainly depends on the quality and dimensions of the iliac and femoral arteries due to the size of the delivery catheters on which the bioprostheses are mounted. Two PAVR systems are currently in clinical evaluation: The Cribier-Edwards aortic valve is implanted through a 22 or 24 Fr arterial sheath (8 and 9 mm external diameter) and the CoreValve Revalving™ System (CRS) can be delivered through an 18 Fr sheath (7 mm external diameter).2, 3
When using the Cribier-Edwards valve, which is a balloon-expandable prosthesis implanted during a short burst of rapid right ventricular pacing (220 min-1), the current practice relies on the surgical removal of the arterial sheath at the end of the procedure.2 The CoreValve device is a self expanding nitinol framed valve that is expanded by slowly pulling back a protective sheath. The implantation technique allows delicate and minute adjustments to certify correct valve positioning.4,5 To-date, the clinical protocol has required a form of cardiac assistance and this has been realised by the use of fem-fem cardiopulmonary bypass (CPB).4,5
One may thus argue the true percutaneous nature of these procedures. Although this may appear petty, certainly when considering the pioneering nature of this treatment which offers a new outlook for patients who until now were denied treatment, the surgical introduction and/or removal of the arterial sheaths (for valve delivery or cardiac support) entails a major clinical burden for the patient. It also encumbers the logistics and applicability of the procedure and prolongs the hospital stay.
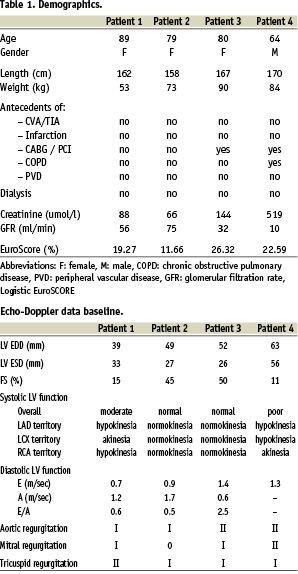
To move towards a full and a truly percutaneous implantation, we are reporting here concerning a new strategy that we have adopted for the CoreValve procedure. It consists of ultrasonic guided punctures of the femoral arteries to allow proper haemostasis with a single Prostar® XL per artery, thus avoiding surgical cut down.6 This strategy is made possible by the use of a TandemHeart® (CardiacAssist) instead of full cardiopulmonary bypass.7
Report
A total of 4 patients underwent full percutaneous aortic valve replacement with the CRS system (demographics, Table 1). The first concerns an 89-year-old female patient without a past medical history except for a hip replacement. She was referred for aortic valve replacement because of rapidly progressive symptoms of dyspnoea due to a severe aortic stenosis (peak velocity of 4.8 m/sec, peak gradient 92 mmHg) in the presence of a moderately impaired systolic left ventricular function (diastolic dimension 39 mm, systolic dimension 33 mm, fractional shortening 15%) without significant aortic and mitral regurgitation (both grade 1) and absence of coronary artery disease.
The physical examination revealed a vital elderly patient (height 162 cm, weight 53 kg) with shortness of breath at rest in the presence of the typical palpation and auscultation of aortic stenosis. There were no signs of left or right heart failure. The blood pressure was 140/90 mmHg. On ECG there was a sinus rhythm (79 beats/min) with normal atrio-ventricular and intraventricular conduction, but left ventricular hypertrophy with strain. Blood count and chemistry were normal except for a glomerular filtration rate of 56 ml/min.
Because of age, renal function and the subsequent risk for neurocognitive dysfunction and renal failure after surgical valve replacement, she was classified as high risk and rejected for surgery (EuroScore: 19,3%). Given the peripheral vasculature, free of atherosclerosis, percutaneous valve replacement with the CRS was proposed for which the patient consented in writing.3
The implantation was performed under dissociated anaesthesia (sedation and analgesia but no intubation and ventilation), which was initiated in the catheterisation suite. A 4 Fr pigtail was advanced via the left radial artery into the ascending aorta for pressure recordings and angiography to guide valve positioning and assessment of the final result.
Next, arterial access was initiated under ultrasonic guidance with a 7.5 mHz linear array probe to insure arterial access at a calcium and plaque free part of the anterior wall of the common femoral artery. This avoided accidental puncture and sheath placement in the superficial femoral artery. A 10 Fr sheath was placed in the left and right common femoral artery. Subsequently on each side one single Prostar® XL 10 Fr system was used to place 2 suture wires. The Prostar system was removed over an 80 cm stiff guide wire and the 10 Fr sheath was replaced. The suture wires were secured with small clamps.
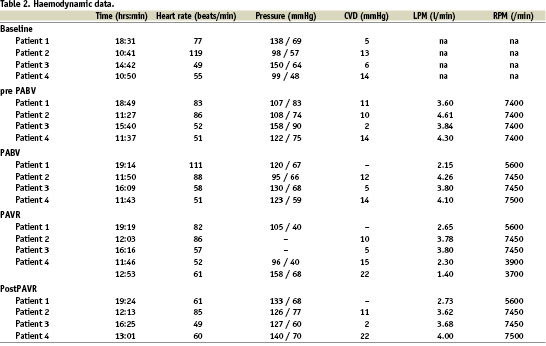
After that, a 12 Fr sheath was inserted in the left femoral vein for the positioning of a 10 Fr AcuNav™ echo catheter (Siemens, Germany) in the right atrium. After clear delineation of the interatrial septum, a transseptal puncture (Brockenbrough technique) was performed via the right femoral vein, followed by the insertion of a 21 Fr venous cannula via the right atrium in the left. The 10 Fr sheath in the left femoral artery was then replaced by a 17 Fr arterial TandemHeart® cannula. After checking the central venous pressure, priming and de-airing of the pump, the TandemHeart® was activated just prior to the balloon aortic valvuloplasty (BAV) that precedes every PAVR procedure. This was accomplished by gradually increasing the rotations per minute (RPM) until an optimal output was achieved. (Table 2 and Figure 1).
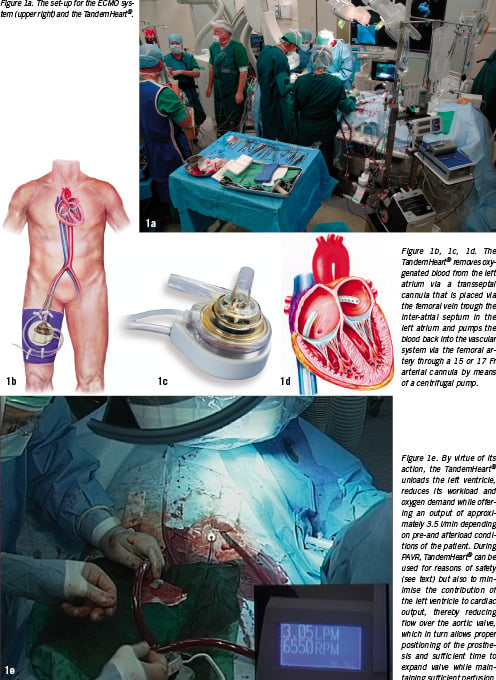
The 10 Fr sheath in the right femoral artery was then replaced by an 18 Fr sheath for the implantation of the aortic valve prosthesis. Crossing of the stenotic valve was performed with a straight Kimal wire. To facilitate the crossing, the RPM’s of the TandemHeart® were set at the lowest level since a fully active pump reduces the already impaired leaflet motion, which may enhance the difficulty of crossing the stenotic valve. The RPM were then reset at the original value and balloon pre-dilatation with a 23 mm balloon was followed by the implantation of a 26 mm inflow size CRS.
After retrieval of the delivery catheter through the 18 Fr sheath, the TandemHeart® was weaned and stopped. The cannulae were clamped, followed by the removal of the arterial cannula from the left femoral artery which was closed straight away by tightening the Prostar® suture wires. Next the 18 Fr sheath was removed from the right femoral artery, which was closed in a similar way. Haemostasis of the femoral veins was obtained by manual compression (Figure 2 a-d). The patient was transferred to the IC/CCU where the anaesthesiologic drugs were stopped. This was followed by extubation and referral to the general ward where she was mobilised the next day in preparation of hospital discharge. The echo before discharge (day 5) revealed a reduction in peak velocity over the aortic valve from 4.8 to 2.0 m/sec resulting in a reduction in the peak gradient from 92 to 16 mmHg. There was a grade 1 aortic regurgitation after valve implantation.
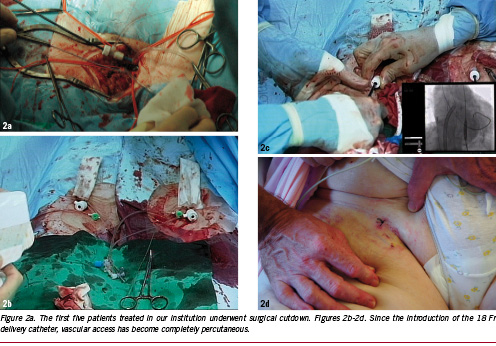
Exactly the same approach was used in three additional consecutive patients who were treated after the patient mentioned above. Of note is that in the last patient, the TandemHeart® was set at a stand-by mode (echo-Doppler data before PAVR, Table 2). The stand-by mode entails the reduction of the RPM to the lowest value possible during valve positioning, and implantation resulting in a minor to insignificant circulatory support (minimal LPM) of the TandemHeart®. The stand-by mode was chosen rather than the clamping of the cannulae to avoid the risk of clot formation.
Discussion
This report summarises the first true, complete percutaneous implantation of the CoreValve aortic valve prosthesis in a series of four patients with aortic stenosis and, as such, heralds the inception of a new era of innovation in cardiovascular medicine. It also marks the consolidation of a dedicated collaboration between biomedical engineering and the industry on one hand, and a multidisciplinary group of physicians prepared to change and translate innovation into reality.
Obviously, one may argue the uniqueness of this reported course of action since the Edwards-Cribier aortic valve prosthesis is implanted during rapid right ventricular pacing of the right ventricle after the percutaneous insertion of a 22 or 24 Fr sheath into the femoral artery and a 5 or 6 Fr sheath in the femoral vein. Yet, these bulky arterial sheaths still require surgical removal, which is no trifling procedure, and may last longer than the implantation of the valve itself, especially in obese patients. In addition to the prolonged hospital stay, possible complications such as infection, delayed wound healing and complicated mobilisation may also result.
The first generation CoreValve aortic valve prosthesis was implanted using a 25 Fr delivery catheter (14 First-In-Man patients: 2004) that was replaced by a 21 Fr (65 Safety and Efficacy Patients: 2005-2006) and currently an 18 Fr (since mid-2006). The initial down-sizing was the result of optimisation of the frame configuration in combination with a new valve design that reduces the tissue mass and bulkiness inherent in surgical type valve designs. Further changes in the technique of suturing of the valve (three leaflets and skirt) into the stent frame, combined with additional catheter space saving manufacturing techniques, led to the current 18 Fr device. Biomedical engineering efforts are currently directed towards a further down-sizing to a 16 Fr system that may become clinically available within the next two years. To what extent the further reduction in size of these systems is possible is not known and is limited by the technologies in use. At present, both the CoreValve and Edwards aortic valve prosthesis use well known – but different – biocompatible metal alloys with known surface interactions and bio-physical and chemical properties such as elastic modulus, tensile strength and corrosion resistance.8-12 For the functional valve parts, both devices also use pericardial materials processed by well-known methods (porcine, bovine and equine). It may well be that the current scaffolding and tissue technologies will be abandoned in favour of novel ones that allow the construction of valve prostheses much smaller in size. For instance, through ion excitement of pure nickel and titanium by argon gas (3 D nanosynthetic stereometric assembly) a very thin e-nitinol stent frame can be produced.13 This technology could also be used to construct ultra thin (10 µm) micro porous metal leaflets with configurable leaflet design for optimal flow dynamics. Consequently, a valve prosthesis may become available that can be implanted through a 10 Fr sheath.13 If this technology fulfils its promises and meets the rigorous clinical criteria and demands, it will have a major impact on the management of patients with aortic stenosis.14
Miniaturisation is one challenge, the other is the technique of implantation. The percutaneous implantation of a stented aortic valve requires precise positioning and expansion of the valve, not only for its proper function but also to respect mitral function and coronary circulation. Cardiac motion and flow effects may impede precise positioning and expansion. In case of the implantation of the Cribier-Edwards aortic valve, cardiac arrest is induced by rapid right ventricular pacing.15 In case of adequate capture, a short burst of pacing at a rate of 220 min-1 sufficiently lowers the systolic and pulse pressure but offers only a tiny time window to expand the stent without any room for adjustments. As mentioned above, the clinical protocol of CRS implantation until recently required some sort of haemodynamic support, which until now, has been achieved by CPB. Although much more cumbersome and invasive, CPB not only offers a time window long enough, as needed, to expand the frame, it also contributes to the safety of the procedure. Rapid ventricular pacing is not a physiologic and reliable approach to reduce blood pressure and flow. It, furthermore, may induce asystole and malignant arrhythmia, such as ventricular fibrillation, that are, in principle, easily correctable, but may cause refractory haemodynamic collapse, especially in patients with structurally changed hearts as a result of age and long lasting pressure overload.15,16 CPB and the percutaneous technique (p-LVAD) used in these present patients, allow continued haemodynamic support prior and after the procedure. These considerations are the reason why we favour circulatory support during these early learning curve percutaneous aortic valve replacement procedures. As demonstrated, introduction and removal of the cannulae can be performed percutaneously. Obviously, this is not without risk. A meta-analysis, disclosed an increased risk of haematoma and pseudo-aneurysm after application of closing devices, something which is a concern both from a clinical or patient perspective as well as an economic and logistic one.17
We prefer p-LVAD such as the TandemHeart® for reasons of logistics, safety and efficacy rather than CPB or related systems. With respect to logistics, the TandemHeart® does not require a perfusionist but is instrumented and controlled by the cardiology-intervention team, which facilitates the procedure and planning. Concerning safety, the TandemHeart® removes oxygenated blood from the left atrium which is then pumped back into the body via the femoral artery. Consequently, no artificial lung is needed and, thus, the blood is exposed to a significantly smaller foreign body surface than when using CPB. This, in combination with other mechanisms of action of the TandemHeart®, minimises the risk of inflammation and related side effects of CPB.18 With reference to safety, the TandemHeart® offers an output of approximately 3.5 l/min, which is adequate enough for the implantation of the valve and also for eventual post-procedural support.19,20 Yet, it is a system that is preload dependent, which can be adjusted by controlling the volume status of the patient, but also depends on right ventricular and pulmonary function and afterload parameters such as the systemic vascular resistance which are less amenable to adjustments.
Yet, as demonstrated in the last patient, it is conceivable not to use either the TandemHeart® or any other circulatory support system in selected patients. This would imply only a one-sided femoral artery puncture leading to a truly minimal invasive or PCI-like aortic valve replacement, eventually performed on an out-patient clinical basis.
We chose to use the TandemHeart® on a stand-by mode in the last reported patient after the experience of Grube et al. who proposed using a short period of induced hypotension or bradycardia, but successfully implanted a CRS system without any support at all on November 6, 2006. This step forward is in line with our observations in previous patients that during positioning and the initial phase of valve expansion there is an adequate amount of flow over the delivery catheter as demonstrated by contrast angiography. During further expansion, the native valve leaflets are pushed aside while the implanted valve takes over the aortic valve function whilst there is a sufficient amount of antegrade flow over the aortic valve area.
Before advocating aortic valve replacement without circulatory support, one should be aware of the risks of haemodynamic deterioration or collapse due to either rhythm disturbances, conduction disturbances or reduced inotropic reserve that may occur during the procedure, balloon predilatation in particular. Unfortunately, this is hard to foretell. Therefore, we propose attempting aortic valve replacement without circulatory support in patients with normal ventricular function, absence of associated valve disease and in a setting in which an experienced team and infrastructure is in place capable of taking swift and appropriate action in case of emergency. We felt comfortable performing valve implantation with the TandemHeart® on stand-by mode in the last described patient, despite his ventricular function, given our experience with the TandemHeart® and valve implantation in a total of nine patients to this date.
In addition to miniaturisation of devices and improvements in circulatory support strategy, one may think of changes in the technique of implantation. It should be faster than the current system and potentially offer the possibility of recapture. Also, one may consider a more sophisticated use of existing imaging techniques in order to construct a cast of the left ventricular outflow tract up to the ascending aorta just beyond the sino-tubular junction which would be useful in defining the configuration of the frame and size of the valve the patient should receive for optimal haemodynamics as well as allowing for a simulation of the implantation before the procedure begins.
In summary, there are currently two systems available for percutaneous aortic valve replacement. Until now, none had been performed truly and completely percutaneously. We reported here on a strategy in which a purely percutaneous aortic valve replacement was successfully accomplished using the CoreValve Revalving System in combination with TandemHeart®.
Online data supplements
Video 1. The valve delivery.
Video 2. Arterial access under echo guidance (short axis).
Video 3. Arterial access under echo guidance (long axis).

