Abstract
Background: Given the poor prognosis of tricuspid regurgitation (TR) patients, there is growing interest in addressing TR, particularly since the emergence of novel transcatheter tricuspid valve interventions for patients at high risk for surgery.
Aims: The TRICURE first-in-human (FIH) study evaluates the initial feasibility and clinical safety of the Topaz transcatheter tricuspid valve replacement (TTVR) system in treating TR. Featuring a novel dual-stent design, the system is specifically engineered for the unique anatomy of the tricuspid valve. It has a flexible outer stent with low radial force designed to accommodate annular dynamics and conform to the non-circular, variable shapes of the tricuspid valve, coupled to a rigid inner stent aiming to maintain valve function integrity.
Methods: TRICURE FIH is a prospective, multicentre, first-in-human study with follow-up extending to 5 years. The primary safety endpoint is a composite measure including all-cause mortality, heart failure rehospitalisation, and reintervention for failed tricuspid therapy at 30 days. The primary performance endpoint is device success, defined as a TR reduction ≥1 grade with no more than moderate TR post-procedure.
Results: A total of 20 patients were enrolled. The procedure time (from delivery system insertion to removal) was 35±16 minutes. At 30 days, the primary safety endpoint, a composite of major adverse events, was observed in 35%, and the primary performance endpoint was successfully achieved in all patients (100%), with all patients having a TR reduction of at least 3 grades, and none of the patients having more than mild TR post-procedure. No device-related pacemaker implant was reported. An exemplary case report demonstrates complete elimination of TR and a reverse remodelling of the right ventricle of 19% at 6 months.
Conclusions: The TRICURE FIH study provides evidence of the feasibility and safety of a novel TTVR system. Outcomes need to be confirmed in a larger series. (ClinicalTrials.gov: NCT05126030)
Once considered the “forgotten valve”, the tricuspid valve has gained increasing attention due to advances in novel treatment strategies for tricuspid regurgitation (TR) and a growing recognition of its prevalence, adverse prognostic impact, and substantial symptom burden associated with progressive right heart failure1. Current guidelines acknowledge the rising interest, supported by encouraging preliminary experience with transcatheter tricuspid valve interventions23. However, the tricuspid valve presents unique anatomical and physiological challenges compared to other heart valves. For example, its large orifice area is highly dynamic, influenced by volume status, while the fragile, three-dimensional D-shaped annulus undergoes substantial changes throughout the cardiac cycle14567. These complexities make the development and implantation of novel transcatheter therapies particularly challenging. Evidence has been published in support of transcatheter tricuspid valve repair; however, certain patient anatomies – such as large annuli, large coaptation gaps, severe tethering, or right ventricular dysfunction – may be better suited for transcatheter tricuspid valve replacement (TTVR). Furthermore, TTVR offers potential advantages such as shorter procedure times, more effective TR reduction, greater procedural reproducibility, and the option for future valve-in-valve procedures78. To date, the EVOQUE valve (Edwards Lifesciences) is the only TTVR system to have received both European Conformity (CE) certification and U.S Food and Drug Administration (FDA) approval, underscoring the need for other TTVR systems. The Topaz TTVR system (TRiCares) has been specifically designed to address the challenges of tricuspid valve replacement. Its innovative dual-stent design consists of a flexible outer stent and a more rigid inner stent. The outer stent features extremely low radial force, designed to minimise the risk of conduction disturbances while seamlessly adapting to the various native annulus morphologies. The inner stent, which houses the trileaflet valve and serves as the “replacement valve”, is designed to maintain a circular shape, thereby ensuring long-term valve integrity and reliable haemodynamic performance. Additionally, atraumatic anchors aim to provide secure and stable fixation below the annulus within the native leaflets (Central illustration, Moving image 1). The TRICURE first-in-human (FIH) study intends to evaluate the feasibility and initial safety of the Topaz TTVR system. This report presents the study’s primary endpoints along with an exemplary case report with 6-month follow-up. 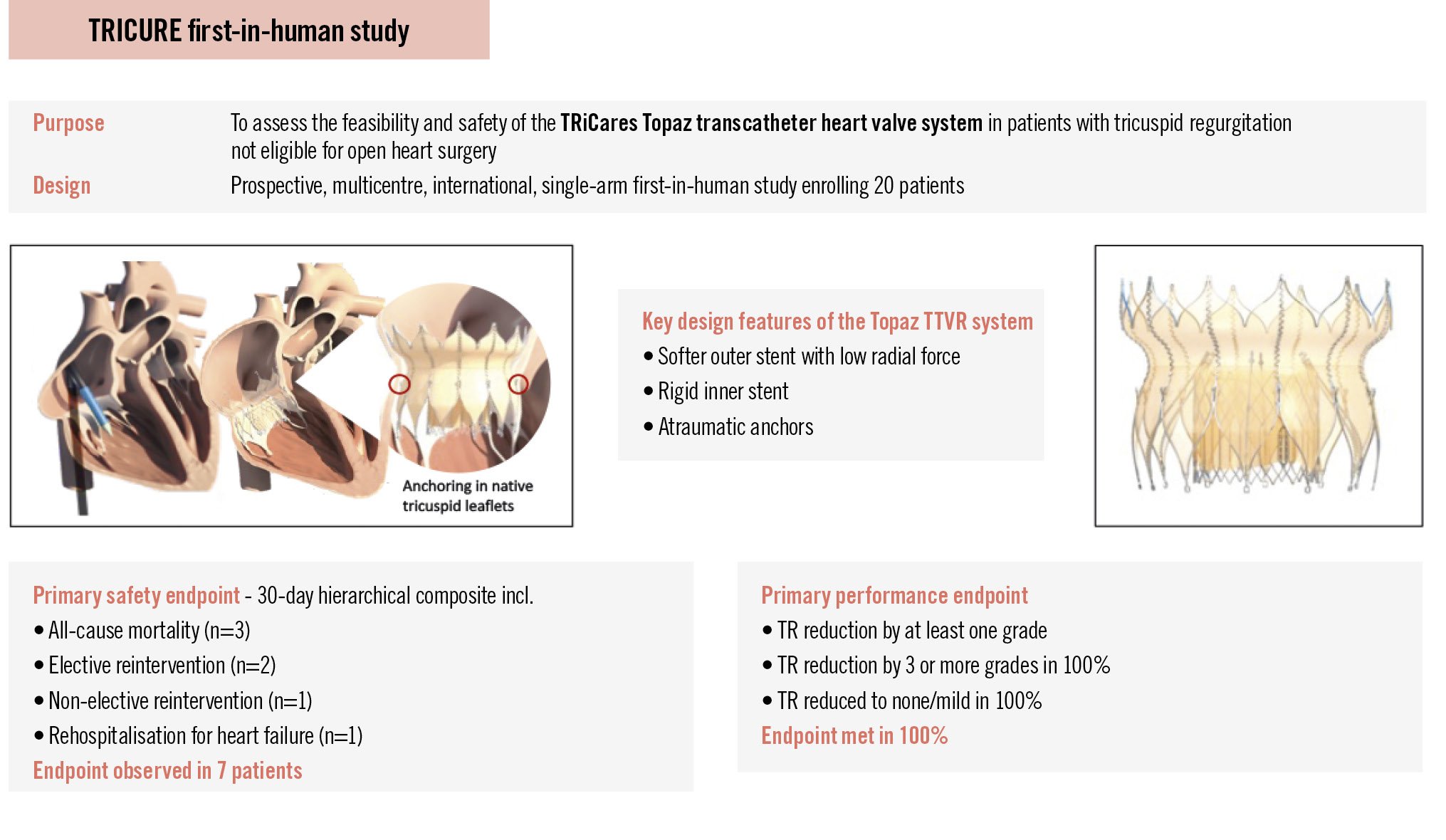
Central illustration. TRICURE first-in-human study assessing the TRiCares Topaz transcatheter tricuspid heart valve system. Data were adjudicated by a clinical events committee and assessed by a core laboratory. TR: tricuspid regurgitation; TTVR: transcatheter tricuspid valve replacement
Methods
Study design
The TRICURE FIH study is a prospective, multicentre, single-arm study conducted at 8 centres in Europe. Clinical follow-up is scheduled at 30 days, 3 and 6 months, and annually at 1, 2, 3, 4, and 5 years. Follow-up assessments include a physical examination, electrocardiography, laboratory tests, echocardiography, a 6-minute walk test, and quality-of-life evaluation using the Kansas City Cardiomyopathy Questionnaire. A computed tomography (CT) assessment will be performed at the 6-month follow-up. Approval was obtained from the competent authorities and all site ethics committees, and all patients provided written informed consent. Study oversight was ensured through an independent clinical events committee that adjudicated all adverse events (including all safety endpoints), an independent data monitoring committee, a screening committee, and independent core laboratories (MedStar Health Research Institute and Georgetown University, Washington, USA, for echocardiography and St. Paul’s Cardiac CT Core Laboratory, Vancouver, Canada, for CT imaging). The trial is registered at ClinicalTrials.gov: NCT05126030.
Patient population
Key inclusion criteria were age above 18 years; TR grade ≥3 (on a 0 to 5 scale)9, as assessed by an independent core laboratory, and/or symptoms requiring diuretic therapy; New York Heart Association (NYHA) Class ≥II; and patients not eligible for tricuspid valve surgery due to high operative risk, as determined by the site Heart Team. Major exclusion criteria included a need for emergent or urgent intervention or any planned cardiac intervention within 12 months; cardiac interventions within 30 days prior to the index procedure; concomitant clinically relevant mitral, aortic, or pulmonary regurgitation or stenosis; prior tricuspid valve replacement or repair with a device in situ; and severe pulmonary hypertension (Supplementary Table 1).
Study device and procedures
The TRiCares Topaz TTVR system comprises four components: (1) the heart valve prothesis; (2) the introducer system; (3) the delivery system for transcatheter access, which together with the introducer system constitutes the implantation system; and (4) the loading system. The Topaz heart valve prosthesis is a self-expanding biological artificial heart valve with a double-stent design consisting of an outer stent and an inner stent, both made of nitinol. The outer stent safely anchors the prosthesis in an orthotopic position by means of an hourglass shape (v-groove) and 12 atraumatic anchors. The inner stent serves as the skeleton for the trileaflet heart valve, which is made of porcine pericardium. The seal, also made of porcine pericardium, is designed to prevent intraprosthetic as well as paravalvular leakage. The available valve size was suitable for native annulus sizes of up to approximately 45 mm. The introducer system consists of a steerable sheath (29 Fr) and a dilator compatible with a 0.035 inch guidewire. The delivery system is designed as a pullback system and has a soft, radiopaque tip aiming to prevent damage to the ventricle, as well as a stent holder designed to securely attach the Topaz prosthesis. The outer shaft maintains the crimped state of the prosthesis until a controlled release is intentionally initiated with the deployment wheel on the delivery system handle. The radiopaque marker band of the outer shaft allows for visual position control under fluoroscopy during deployment. An animation of TTVR with the Topaz system is provided as Moving image 1. Baseline assessments included transthoracic and transoesophageal echocardiography, CT, and right heart catheterisation. Anticoagulation therapy was to be prescribed according to hospital standard of care for heart valve replacement and/or following the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) Guidelines for the management of valvular heart disease2.
Endpoints and definitions
The primary safety endpoint is a composite endpoint measure that includes all-cause mortality, rehospitalisation for heart failure, and reintervention for failed tricuspid intervention at 30 days. In cases where the patient was still in hospital on day 30 post-intervention, this was considered a rehospitalisation. The primary performance endpoint is device success, defined as a reduction of tricuspid regurgitation by at least 1 grade between the baseline and post-intervention evaluation (as assessed by the echo core laboratory), and no more than moderate tricuspid regurgitation. Secondary clinical endpoints are listed in the results section10. Functional and quality-of-life endpoints, along with comprehensive imaging analysis covering all follow-up timepoints, will be reported in a separate publication.
Statistics
A sample size of 20 patients was deemed to be adequate to get first data on feasibility, safety, and performance of the TRiCares Topaz TTVR system. Safety outcomes are reported for the intention-to-treat population. Procedure time was calculated based on the patients in whom the delivery system was introduced. Performance analyses were done for all patients with the investigational device in situ. Patients who did not receive a study valve or had a study valve explanted were followed for 30 days for safety assessment. Continuous data are summarised as mean±standard deviation (minimum and maximum). Categorical variables are summarised as frequency counts and percentages. The statistical analysis was performed using SAS, version 9.4 (SAS Institute).
Results
Patients who were deemed ineligible for open heart surgery were enrolled; none of the 8 sites enrolled more than 20% of patients (maximum of 4 patients per site).
Baseline characteristics
Twenty patients were enrolled, with a mean age of 77.0±6.6 years, and 18 patients (90%) were female. Baseline characteristics are presented in Table 1.
Table 1. Baseline characteristics.
| N=20 | |
|---|---|
| Age, years | 77.0±6.6(61-81) |
| Sex | |
| Male | 2 (10) |
| Female | 18 (90) |
| NYHA Class | |
| II | 6 (30) |
| III | 13 (65) |
| IV | 1 (5) |
| EuroSCORE II, % | 4.2±2.3(1.3-10.2) |
| STS-PROM score, % | 9.7±5.4(4.6-22.1) |
| TRI-SCORE | 4.6±2.5(2-11) |
| TRI-SCORE predicted in-hospital mortality, % | 17.6±20.1(3-65) |
| Concomitant mitral valve disease | 10 (50) |
| Concomitant aortic valve disease | 3 (15) |
| Coronary artery disease | 4 (20) |
| Cardiac rhythm | |
| Bundle branch block | 4 (20) |
| Atrial flutter/fibrillation | 17 (85) |
| History of previous CIED implantation | 0 (0) |
| Previous stroke | 5 (25) |
| Peripheral vascular disease | 3 (15) |
| Chronic obstructive pulmonary disease | 2 (10) |
| Renal insufficiency* | 19 (95) |
| eGFR, mL/min/1.73 m² | 40±19 |
| Pulmonary hypertension | 9 (45) |
| Pulmonary artery systolic pressure, mmHg | 39.8±13.1 |
| Cancer | 6 (30) |
| Diabetes | 5 (25) |
| Arterial hypertension | 15 (75) |
| Hyperlipidaemia | 13 (65) |
| Smoker | 6 (30) |
| Data are presented as mean±SD (min-max), mean±SD, or n (%). *eGFR of ≤89 mL/min/1.73 m2. CIED: cardiac implantable electronic device; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | |
Procedural outcome
The Topaz valve was implanted in 18 patients. The mean procedure time was 35±16 min (range 17 to 65 min), and the mean right atrial pressure decreased from 21±13 mmHg preimplant to 12±5 mmHg post-implant. Two patients did not receive the device due to access-related challenges. In one patient, venous access was not achievable because of a thrombotic vessel, which was a result of long-term dialysis. The other patient had a rupture of the access vessel that was due to the vessel’s frailty after radiation therapy. Both patients were alive at 30 days post-procedure. Since then, the screening committee has also begun assessing the access vessels with particular care, recognising that even though the venous system may appear more forgiving, it still warrants a thorough evaluation to ensure that transcatheter access can be achieved. One patient experienced procedural mortality due to a ventricular perforation. It cannot be ruled out that the stiff guidewire may have contributed to the ventricular perforation, particularly given the known fragility of the right ventricular wall in transcatheter interventions. The patient underwent emergency surgery, but died the same day. In another patient, the stiff guidewire inadvertently became wrapped underneath the moderator band. This detail went unnoticed, leading to deployment of the Topaz valve above the moderator band, which resulted in a suboptimal position that ultimately required surgical reintervention. Thirty-day data are available for all patients. The primary safety endpoint, a composite of major adverse events (MAE), was observed in 7 patients (35%) (Table 2). In two patients, perpendicular alignment of the delivery system could not be achieved, resulting in the prothesis being deployed slightly higher than intended. In one of these cases, the nose cone of the delivery system (catheter tip) became entrapped in the prothesis during retrieval, causing valve displacement. As the valve was not securely anchored, the implantation team reached a consensus to proceed with an elective reintervention on the same day, during which the Topaz prosthesis was successfully secured with a few sutures. The patient showed initial improvement but subsequently developed cardiogenic shock and right ventricular failure, passing away on day 5. In the other patient, perpendicular deployment proved difficult because of a small, short right ventricle. Although the Topaz valve initially appeared well-seated, postprocedural migration – likely caused by upward pressure from subvalvular structures, including a dominant papillary muscle and trabeculations – was detected on day 2, prompting elective replacement with a conventional valve. The Topaz valve was explanted at the surgeon’s discretion rather than secured with sutures. The patient was alive at 30 days. In a third patient, the procedure was uneventful, with initially correct valve placement. Shortly after, the valve migrated slightly posteriorly but remained stable. Echocardiography showed mild to moderate paravalvular regurgitation. Root cause analysis revealed an underappreciated posterior shelf that impaired complete anchoring, with a subannular diameter of 58 mm likely exceeding the valve’s anchoring capacity. In an elective reintervention on postinterventional day 1, the valve was successfully secured with a few sutures. As a result, screening was refined to include more detailed assessment of annular and right ventricular dimensions. In addition to the two patients described above, one patient – an 80-year-old with multiple comorbidities and a recent history of catheter-related sepsis – died of septic shock and mesenteric ischaemia 19 days post-procedure. The patient had received levosimendan both pre- and post-intervention and was on haemodialysis. The infection was likely linked to a previously infected access site; endocarditis was ruled out. No Topaz-related pacemaker implantation was reported. The primary performance endpoint was achieved in all patients (100%) with a device in situ. In fact, TR was reduced by at least 3 grades in all patients (100%). At 30 days, tricuspid regurgitation was absent in all but two patients, both of whom presented with mild TR (Figure 1). Independent core lab analysis of paired transthoracic echocardiograms showed that, from baseline to 30 days, right atrial volume decreased from 75.8±22.1 mL to 62.3±13.3 mL, inferior vena cava (IVC) diameter decreased from 19.1±4.5 mm to 17.4±2.4 mm, tricuspid annular plane systolic excursion (TAPSE) decreased from 18.6±6.1 mm to 16.3±5.0 mm, and right ventricular fractional area change decreased from 36.0±8.7% to 29.4±9.6%.
Table 2. Clinical outcomes at 30-day follow-up.
| N=20 | |
|---|---|
| Hierarchical composite MAE endpoint* | 7 (35) |
| All-cause mortality | 3 (15) |
| Cardiovascular mortality | 2 (10) |
| Non-cardiovascular mortality | 1 (5) |
| Reintervention# | 3 (15) |
| Elective (Topaz remained in situ) | 2 (10) |
| Non-elective | 1 (5) |
| Hospitalisation for heart failure | 1 (5) |
| Additional safety endpoints | |
| Myocardial infarction | 0 (0) |
| Stroke | 0 (0) |
| Renal complications | 0 (0) |
| Vascular bleeding | 0 (0) |
| Device-related pulmonary embolism | 0 (0) |
| Topaz-related pacemaker implantation† | 0 (0) |
| Thrombotic event | 0 (0) |
| Data are presented as n (%). *Includes all-cause mortality, rehospitalisation for heart failure, and reintervention for failed tricuspid intervention. #N=1 explanted; N=2 Topaz prosthesis remained in situ. †None of the enrolled patients had a prior pacemaker implant. MAE: major adverse events | |
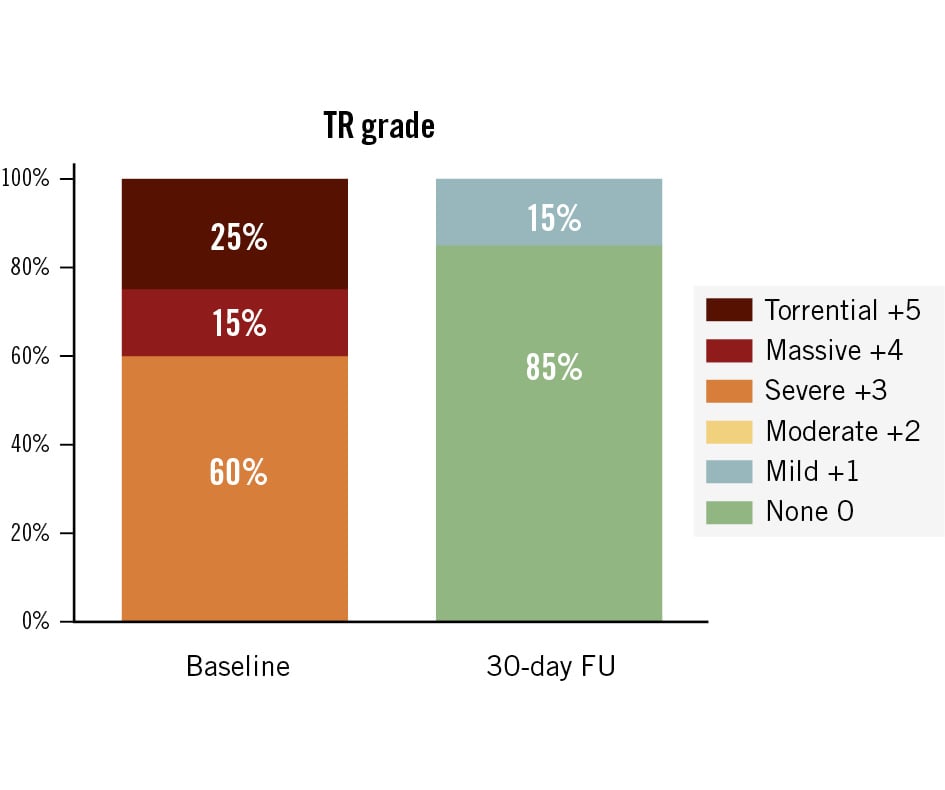
Figure 1. Tricuspid regurgitation at baseline and 30-day follow-up. Data were assessed by a core laboratory and were available for 20 patients at baseline and 13 patients at 30-day follow-up. Each patient improved by at least 3 grades at 30 days. FU: follow-up; TR: tricuspid regurgitation
Exemplary case report
We report the case of a frail 78-year-old female with New York Heart Association (NYHA) Class III symptoms and massive TR grade 4/5 due to annular dilatation in the setting of permanent atrial fibrillation, a retracted septal leaflet, and clefts in both the posterior and anterior leaflets (Moving image 2-Moving image 3-Moving image 4). The core laboratory measured the tricuspid annulus as 41 mm on echocardiography, while CT assessment in end-systole showed dimensions of 42 mm × 45 mm in orthogonal planes. The TAPSE was 15 mm. The patient experienced fatigue and exertional chest pain; she had a TRI-SCORE of 4 out of 12, resembling a predicted in-hospital mortality of 8%, and a Society of Thoracic Surgeons (STS) score of 9.7%. Further details are provided in Figure 2. Her multiple comorbidities included aortic insufficiency, mild mitral insufficiency, arterial hypertension, permanent atrial fibrillation, left bundle branch block, moderate pulmonary hypertension, a history of decompensated heart failure (baseline N-terminal pro-brain natriuretic peptide [NT-proBNP] of 2,372 pg/mL and cardiac output of 3.0 L/min), renal insufficiency (baseline estimated glomerular filtration rate of 44 mL/min/1.73 m²), and previous left mastectomy and radiotherapy. Right heart catheterisation revealed a pulmonary artery pressure of 40/20/26 mmHg, a pulmonary capillary wedge pressure of 16/20 mmHg, and a right atrial pressure of 15/19 mmHg. The procedure was performed under general anaesthesia. Access was gained through the left femoral vein. The CT scan was used to define the implant angulation and the spatial relationship between the right coronary artery and the tricuspid annulus. Via the steerable sheath, the valve was advanced towards the right ventricle. After achieving perpendicular alignment of the delivery system with the tricuspid annulus and confirming the correct height at the annular level, a bottom-to-top release was performed under fast pacing (Moving image 5-Moving image 6-Moving image 7-Moving image 8). The procedure time (from delivery system insertion to removal) was 28 min, and TR was successfully and completely eliminated (grade 0/5) (Moving image 9-Moving image 10-Moving image 11). Two adverse events were observed. Following successful valve implantation, the patient developed a complete heart block requiring temporary pacing, and the temporary pacemaker was safely removed on postoperative day 2. Notably, the patient had a pre-existing left bundle branch block. Additionally, the patient experienced a transient post-anaesthesia delirium, with a normal neurological examination and a negative brain CT. The condition resolved fully by postoperative day 2, without any residual confusion or disorientation. The patient was discharged in a stable condition on postoperative day 10. At 30 days, and again at 3 and 6 months, the patient showed a sustained and remarkable improvement to NYHA Class I, with no recurrence of TR and a restored TAPSE of 19 mm. By 3 months post-procedure, diuretics were no longer needed. At 6 months, the CT scan revealed a 19% reduction in right ventricular end-diastolic volume, evidence of reverse remodelling, and ongoing cardiac recovery. 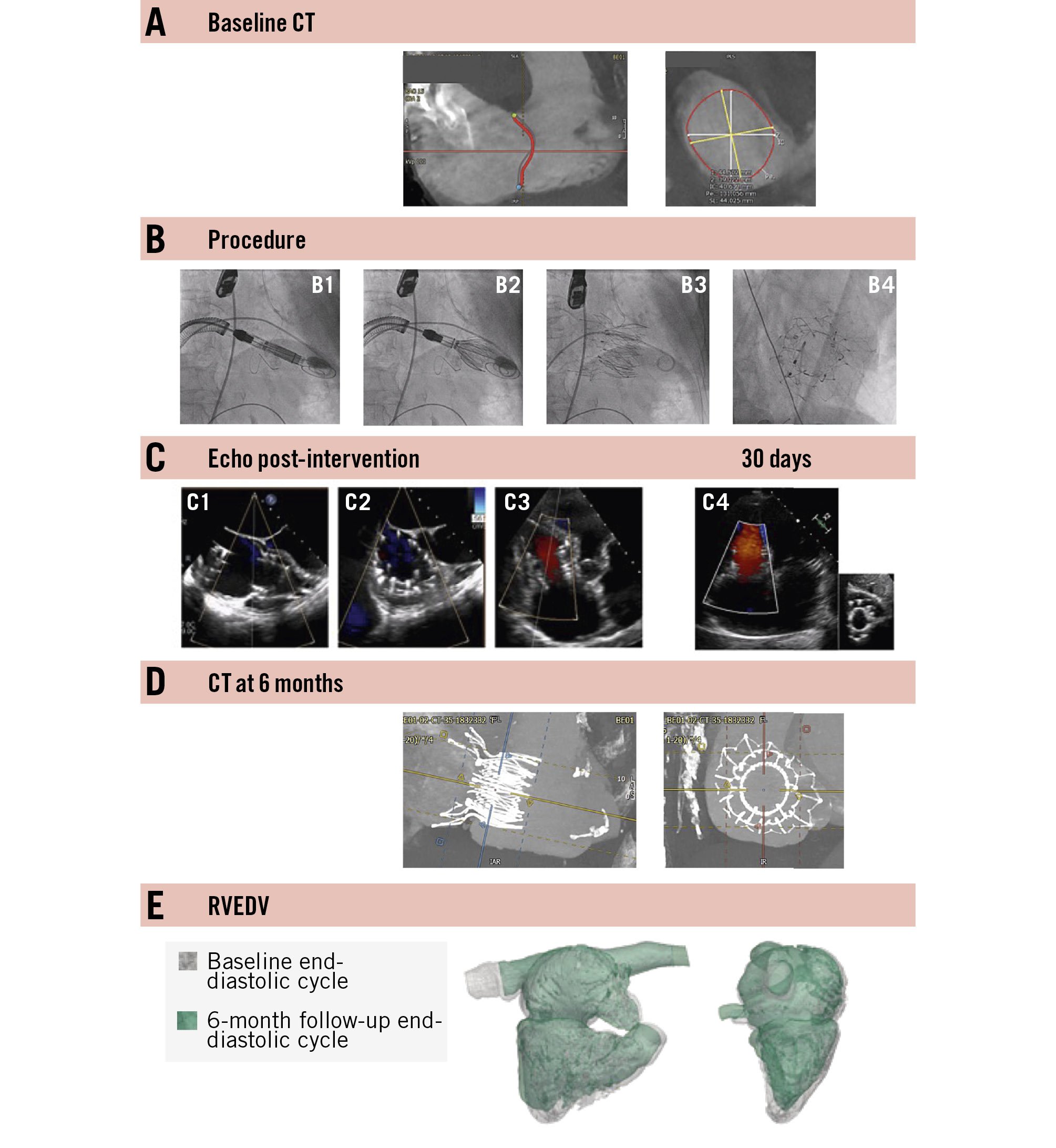
Figure 2. Exemplary case report with echocardiographic and computed tomography assessments. Exemplary case report of a 78-year-old female. A) Baseline computed tomography (CT) revealed an oval-shaped annulus in systole, delineated by a red line, with a minimum diameter of 42 mm and a maximum diameter of 45 mm. B1) Using a steerable sheath, the valve was advanced towards the right ventricle. The wire in the right coronary artery served as a landmark for the tricuspid annulus during positioning. B2) The delivery sheath was partially retracted, allowing for the assessment of valve height and perpendicular alignment with the annulus using multiplanar transoesophageal imaging. B3) After confirming the valve position, the valve was deployed from bottom to top under fast pacing. B4) Final angiography demonstrated excellent results: no residual leakage, a circular inner ring/stent, and an outer ring well-conformed and adapted to the anatomical annular shape in the en face view. C1-C3) Postprocedural results by transoesophageal echocardiography and (C4) transthoracic echocardiography on day 30 post-procedure. D) CT imaging at the 6-month follow-up confirmed the persistent round shape of the inner ring and continued conformity of the outer ring to the annulus. E) The right ventricular end-diastolic volume (RVEDV) decreased by 19%, from 175 mL at baseline to 142 mL at 6 months, as assessed by the core laboratory.
Discussion
The main findings of this FIH series are as follows: (1) acceptable safety, with an MAE composite rate of 35%, (2) low technical and procedural complexity, as reflected by a mean procedure time of 35±16 minutes; (3) a substantial and sustained reduction in TR at 30 days in all patients, accompanied by early signs of reverse right heart remodelling, illustrated by the exemplary case; and (4) a procedural learning curve, which initially led to some elective reinterventions and ultimately drove refinement of screening criteria and optimisation of the implantation technique, including the adoption of additional access routes.
Feasibility and design objective
The Topaz TTVR system is uniquely designed for the tricuspid space, featuring a double-stent system. The TRICURE first-in-human study confirms that the design objectives were met. First, ease of use is supported by the design features of the Topaz valve, such as the hourglass shape and v-groove, which promote intuitive positioning and partial self-alignment. Importantly, the system does not require leaflet grasping or capture prior to deployment, as is necessary with some other transcatheter tricuspid valve systems111213. Valve deployment is guided by transoesophageal echocardiography, although in select cases transthoracic echocardiography may be sufficient, making the imaging requirements less demanding than for some other devices111213. In the TRICURE FIH study, ease of use was reflected by the very short mean procedure time of 35±16 min (from delivery system insertion to removal), despite all centres being first-time users and only enrolling a maximum of four patients each. For comparison, the EVOQUE valve, the first valve that gained CE certification and FDA approval, demonstrated procedural times of 71.6±31.4 min in TRISCEND and 56.5 min (interquartile range 41-75 min) in TRISCEND II1415. Second, the conformity of the outer stent and its sealing are intended to prevent paravalvular leakage and rhythm disturbances that may result in permanent pacemaker implantation. This has been confirmed by the successful reduction of TR at 30 days and the fact that there were no Topaz-dependent permanent pacemaker implants at 30 days, compared to 13.3% in TRISCEND and 24.7% in TRISCEND II1415. As shown in the exemplary case, the conformity of the outer stent also prevents imposing a round valve shape on the annulus and permits the outer stent to flex and adapt during the cardiac cycle, which may prevent thrombotic events and has the potential to lower the need for anticoagulation therapy. Third, the rigid inner stent helps maintain the valve's integrity. While 30-day outcomes are too early to draw any conclusions about long-term efficacy, the fact that all patients experienced no or mild TR, along with the round shape of the inner stent, the absence of TR in the exemplary case at 6 months, and evidence of reverse remodelling, all provide initial support for the device concept’s potential for success. Fourth, the atraumatic anchoring is designed to prevent annular injury. No such injury was observed in the TRICURE FIH study. However, these complications are rare, and the sample size may be too small to draw a valid conclusion on this aspect.
Insights
This first-in-human study included first-time users, with each centre enrolling a maximum of 20% of the total patients (n=4). Thus, the learning curve includes both individual practitioner experience and the development of effective screening and implantation strategies. While the number of reinterventions, including both elective and non-elective procedures, was notable, this early experience provided valuable insights into patient screening, including vascular access, device sizing, subvalvular structures, and IVC--to-annulus offset (IVC offset), all of which contributed to the challenging tricuspid valve morphology. As expected in FIH studies, suboptimal outcomes were not tolerated and required reintervention. All reinterventions, except two in which the investigator preferred to use a conventional heart valve, were successfully managed by repositioning and securing the Topaz prosthesis with a few sutures. In these cases, the minimally invasive procedure was faster and less invasive compared to conventional open heart surgery. Root cause analyses identified various contributing factors, with sizing and IVC offset emerging as key issues. Subsequently, the implantation guidelines were updated, particularly regarding valve sizing and inclusion of left femoral vein access to address the IVC-to-annulus angle (offset), which allows for a more central position of the delivery system. The development of a larger valve size – already successfully tested in humans – enables the inclusion of more patients with larger annulus dimensions. Another key lesson learned is the importance of screening access vessels, even when venous access is planned. In retrospect, the two patients with failed access should have been excluded during screening.
Outline
There is still much to learn, and these findings, particularly the updated implant and sizing recommendations, will need validation in larger patient populations with long-term follow-up. The follow-up of the current study is scheduled for up to 5 years, and as of manuscript preparation, two clinical trials are ongoing: a European pivotal study (TRICURE EU Pivotal Study [ClinicalTrials.gov: NCT06581471]) and an early feasibility study (EFS) in the USA and Canada (TRICURE EFS [ClinicalTrials.gov: NCT06506942]). The outcomes of these studies may help to define the role of this novel system within the current landscape of tricuspid regurgitation therapies. Provided that the number of reinterventions will be reduced with updated screening guidelines, high-risk patients with multiple comorbidities, patients with renal impairment, and patients with end-stage heart failure might benefit from the shorter procedure times, which may also improve cost-effectiveness. Additionally, patients with pre-existing conduction disturbances who do not yet require a pacemaker may benefit from this device. The findings of this FIH study indicate that Topaz might be a viable treatment option for a vast majority of patients, given its design, which is specifically tailored for the tricuspid valve.
Limitations
The TRICURE FIH study has several inherent limitations typical of FIH trials. These include a learning curve, with each site enrolling a maximum of 4 patients; amended implantation and screening guidelines; and a non-randomised design along with a small sample size, limiting the ability to draw meaningful comparisons with other devices; and lastly, only one prosthesis size was available for the study, though a second size is currently being tested. A positive aspect is the rigorous study conduct, with no patient lost to follow-up.
Conclusions
The Topaz TTVR system is an innovative technology that shows considerable potential in achieving its intended design objectives. If the updated screening and implantation guidelines effectively reduce the number of reinterventions observed in this early experience, the Topaz TTVR system has the potential to offer a user-friendly solution with short procedure times, low complication rates, particularly pacemaker implantations, and effective elimination of tricuspid regurgitation. However, long-term follow-up and data from a larger patient population are essential to validate these results and confirm the safety and efficacy of the revised implantation strategy.
Impact on daily practice
The TRICURE first-in-human study demonstrated the feasibility of the Topaz transcatheter tricuspid valve replacement (TTVR) system, featuring an innovative double-stent design. Although initial procedural challenges led to refinements in screening and implant technique, the consistently short procedure times, absence of device-related pacemaker implantation, and successful elimination of tricuspid regurgitation in nearly all patients support the device design and position the valve as a promising alternative to existing technologies. These encouraging results have led to the launch of two studies aiming to further evaluate the Topaz TTVR system in a broader patient population.
Acknowledgements
We thank Beatrix Doerr, medical writer, for her help in preparing this manuscript.
Funding
The study was funded by TRiCares GmbH, Aschheim/Munich, Germany.
Conflict of interest statement
L. Rosseel reports institutional research grants from Medtronic; consulting fees from GE HealthCare; proctor fees from TRiCares; and speaker fees from Medtronic and Boston Scientific. S. Verheye reports consulting fees from Shockwave Medical; and honoraria from Elixir Medical. J.-F. Obadia reports participation in DSMB/advisory board meetings of Trinity (Jenscare). F.M. Asch directs an Academic Echocardiography Core Laboratory with institutional research grants/contracts from TRiCares, Abbott, Edwards Lifesciences, Boston Scientific, Medtronic, VDyne, CroiValve, InnovHeart, Polares Medical, Ancora Heart, Xeltis, Neovasc, Foldax, CorFlow, Laminar, Aria CV, Us2.ai, Ultromics, GE HealthCare, Philips/Tomtec, egnite, and MyCardium; and is a board/committee member of the American Society of Echocardiography and the American Heart Association. P. Blanke is a consultant for TRiCares, Laralab, and Edwards Lifesciences; and reports stocks/stock options from Laralab and TRiCares. J. Dreyfus reports consulting and/or proctoring fees from Abbott, Edwards Lifesciences, and TRiCares. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animated visualisation of transcatheter tricuspid valve replacement with the Topaz transcatheter heart valve system (video provided with permission of TRiCares, Germany).
Moving image 2. Baseline transthoracic and transoesophageal echocardiography.
Moving image 3. Baseline transthoracic and transoesophageal echocardiography.
Moving image 4. Baseline transthoracic and transoesophageal echocardiography.
Moving image 5. Procedural steps under fluoroscopy.
Moving image 6. Procedural steps under fluoroscopy.
Moving image 7. Procedural steps under fluoroscopy.
Moving image 8. Procedural steps under fluoroscopy.
Moving image 9. Immediate postprocedural results by transoesophageal echocardiography.
Moving image 10. Transthoracic echocardiography on day 3 post-procedure.
Moving image 11. Transthoracic echocardiography on day 6 post-procedure.

