Randomised controlled trials (RCTs) are the gold standard by which the efficacy and safety of diagnostic and therapeutic strategies are evaluated1. Randomisation means that trial participants have a predefined probability of assignment to one of the predefined allocation groups. The allocation should neither be predictable nor determined by the researcher, treating physician or participants. Randomisation results in valid estimates of treatment effect, as it minimises bias due to differential selection and confounding2. The quality of a RCT, however, also depends on another important aspect of RCTs, namely blinding. Blinding refers to study related procedures after randomisation and aims to keep those who are involved unaware of the assigned treatment. Blinding is mainly incorporated in RCTs to assure validity by reducing the subjective expectation of participants (information bias), healthcare providers and researchers and preventing these expectations from influencing the findings3.
As indicated above, adequate randomisation is expected to equalise differences between treatment groups. In most study reports potential confounders are presented in a table displaying baseline characteristics in order to provide readers with information on the comparability of the treatment groups4. The unique feature of randomisation is that unknown confounders are assumed to be equally distributed among the groups as well. Although it is to be expected that adequate randomisation results in the absence of differences between the groups, this is no guarantee and may reflect differences due to chance. Especially in studies with limited numbers of patients an imbalance in some variables may occur by chance only.
Before initiating a RCT, it is important to generate the random allocation sequence (Figure 1). Although today this is most often done by using computer algorithms, statistical textbooks provide tables that can also be used. The three most commonly used types of randomisation are:
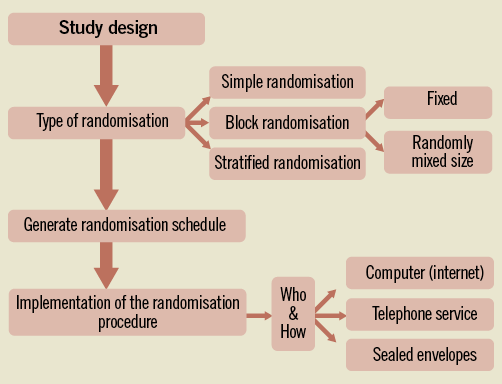
Figure 1. Steps in the randomisation process.
1. Simple randomisation, a method equal to tossing a coin for every single participant entering a trial. This type of randomisation is simple and easy to implement. It should be noted, however, that, particularly in smaller studies (n <100), the distribution of trial participants among the groups may be unequal.
2. Block randomisation, to ensure equal treatment allocation within each block (of fixed or randomly mixed size), and useful in preventing differences between groups based on changes in provided health care during the course of a trial and/or multiple participating hospitals. A disadvantage of fixed block sizes is that the allocation of participants may become predictable, and consequently introduces selection bias. In order to prevent this, it is essential that the investigators remain blinded to the block size. In addition, blocks of randomly mixed sizes may help to avoid predictable allocation.
3. Stratified randomisation, useful when an imbalance in prognostic variables is expected which may, if not balanced between the treatment groups, affect the outcome. These variables, however, need to be identified in advance of preparing the randomisation strategy. In addition, the number of variables included in this randomisation strategy is limited, as too many block variations may result in many blocks with only limited number of patients and, consequently, imbalance in the allocation of participants.
The quality of randomisation is related to the strict implementation of unpredictable allocation, also referred to as “adequate allocation concealment”. In particular, the validity of the findings may be discussed when there is (evidence of) inappropriate allocation concealment. Patients, for example, may have been deliberately included or excluded in a trial when the allocation group is known in advance (e.g., opening or deciphering envelopes or predictable allocation due to a fixed sequence, for example ABBAABBA), thereby introducing selection bias. In this context, it is important to note that using sealed envelopes is more susceptible to manipulation than using a telephone or computer for the allocation5. Importantly, trials with inadequate or unclear concealment have shown a more beneficial treatment effect as compared to adequate concealment5,6, and thus may influence the correct interpretation of a trial. Consequently, and as included in the Consort statement (Table 1), when preparing a manuscript, authors should provide adequate information on the randomisation procedure including information on how (successful) treatment allocation was initiated7.
With regard to the randomisation procedure itself, it is also important to define how the randomisation will be implemented. This should include information on who is authorised to randomise, how patients are randomised (e.g., by telephone, computerised or via sealed envelopes), and at what time-point (for example, immediately after enrolment or just prior to the intervention in the cathlab).
In the context of patient safety and how to handle in case of emergency, healthcare providers must be able to know what treatment allocation a patient was subject to. For this reason it is important to include a procedure on when and how to break the randomisation code.
Key elements of successful randomisation:
– Allocation sequence is prepared in advance
– Allocation sequence must be unknown and unpredictable
– Allocation may not be determined by investigators, clinicians or participants
– If possible, treatment allocation is blinded to the investigators, participants and evaluators
Conflict of interest statement
The authors have no conflicts of interest to declare.

