Abstract
Background: Contemporary stents discharge nickel, chromium and molybdenum which might contribute to restenosis. The Titan stent is coated with titanium nitride-oxide that prevents completely discharge of metal elements.
Aims: To assess short and long term outcome of the Titan in a multi-centre registry.
Methods: Included were all patients except those in cardiogenic shock. Titan stents were 2.0-5.0 mm in diameter and 7-28 mm in length. Clinical follow-up was performed at 30 days and 6 months.
Results: Total of 333 Titan stents were implanted in 296 patients (age 68.8±11.8 years, 81% men). Risk factors included hypercholesterolaemia (61.3%), hypertension (51.3%), diabetes mellitus (DM) (36.6%) and current smoking (27.6%). Eighty-one percent of patients had Acute Coronary Syndrome (ACS). Sixty two percent of treated lesions were B2/C type. Lesion length was 17.5±14.8 mm and stent diameter was 3.0±2.12 mm.
Procedural success was 99.7%. At 180 days, 6.3% of patients had a total of 7.6% MACE including 5.4% TLR, 0.7% MI, 0.7% stent thrombosis and 0.7% death.
Conclusion: The Titan stent has a remarkable safety profile. Notwithstanding the highly complicated lesion and case mix, the short- and long-term results of this registry approach those of drug-eluting stents.
Introduction
Inflammation is an imperative factor in the pathogenesis of restenosis. Contemporary stents are made of stainless steel or cobalt/chromium alloys, all of which comprise significant amounts of nickel, cobalt and chromium and some contain also molybdenum. Up to 15% of the population have an allergy to nickel, chromium or molybdenum, triggered by reaction of inflammatory cells to metals charged with ions. Such allergies might therefore contribute to restenosis.
The Titan stent (Hexacath Company, France) is a balloon expandable stainless steel stent coated with titanium nitride-oxide which prevents completely discharge of nickel, chromium and molybdenum. Windecker et al have shown that Titanium-Nitride-Oxide (TiNOX) coating diminishes cell and tissue reaction in a pig model1. We have found exceptionally good outcomes after implantation of a Titanium-Nitride-Oxide (Titan) stent in a small group of patients2. Recently, a small multi-centre randomised clinical study has demonstrated reduction in restenosis by the TiNOX stent compared to stainless steel stents3. To assess short and long term outcome of the Titan stent we performed a multi-centre registry of Titan stent implantation.
Methods
Patients
This multi-centre (9 hospitals) registry was performed between April 1, 2003 and January 31 of 2005. The first 100 patients were recruited as a part of the requirements of the Israeli Ministry of Health for approval of new stents. The registry was then extended by recruiting another 196 patients in 2 medical centres (Hadassah, Jerusalem and Tel-Aviv hospital, Tel-Aviv). Included were all patients who were candidates for stent implantation. Since most operators excluded patients in cardiogenic shock from national new stent registries, we excluded such patients from this paper.
Lesions
Lesions treated with the Titan stent included de-novo and restenotic lesions of native coronary arteries and Saphenous Vein Grafts (SVG).
Stents
Titan stent diameter was 2.0-5.0 and length was 7-28 mm.
Data analysis
Clinical data included gender, age, risk factors, cardiac history and clinical presentation. PCI data included lesion location, type and characteristics, delivery data and deployment pressure. Vessel diameters and lesion length were estimated by the operators.
Thirty day and 6 month phone-call follow-up was performed by an independent research nurse. Outcome data included immediate technical and clinical success and complications, and major adverse cardiac events (MACE) including sub-acute thrombosis, MI, target lesion revascularisation (TLR), target vessel revascularisation (TVR) and death, at 30 days and 6 months.
Results
Patients’ demographics
A total of 333 Titan stents were implanted in 296 patients. Six month follow-up was completed in 295 patients (one tourist patient was lost to follow-up).
Patients’ age was 68.8±11.8 (33-87) years and 246 (81%) of them were men. Risk factors for coronary artery disease, previous cardiac history and indications for PCI are presented in Table 1.
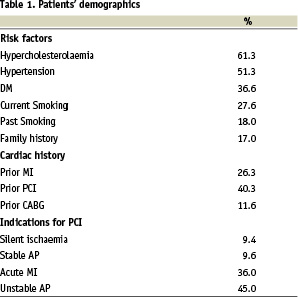
Coronary anatomy and lesion demographics
Thirty-three percent of the patients had single vessel, 30% had double vessel and 37% had triple vessel disease. The vessels treated, lesion location, lesion type and indications for stenting are presented in Table 2.

Stent diameter was 3.0±2.12 mm and lesion length was 17.5±14.8 mm. Fifty-one percent of the lesions were less than 15 mm in length, 36% were 15-20 and 13% were 15-20 mm in length.
Stent deployment and peri-procedural medical treatment
In 91% of the patients dilation of the lesion with a balloon catheter was performed prior to stent implantation and in 9% direct stenting was performed. This reflects the practice of the operators during the registry period. The pressure used for deployment was < 12 bar in 21% and > 12 bars in 79% of the patients.
All patients were treated during PCI with heparin, and 28% were treated with IIb-IIIa antagonists. All patients were treated with clopidogrel 75 mg for one month and aspirin 100 mg for life.
Angiographic and clinical outcome
Procedural success was 99.7%.
At 30 days 1% of patients (3 patients) had total of 1.6% MACE, including 0.6% TVR (Table 3).
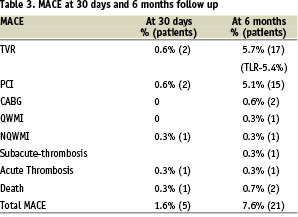
At 180 days 6.3% of patients had total of 7.6% MACE including TVR 5.7% (5.4% of them TLR), 5.1% of which were PCI and 0.6% CABG (Table 3). One patient underwent Non-Q MI due to acute thrombosis and another patient Q-wave MI due to sub-acute thrombosis (total of 0.7%). Two patients (0.7%) died; the first, a 74 year old lady, had a Titan stent implanted to Cx and a non-Titan stent to SVG to PL. Because of no-flow in the PL she was treated with IIb-IIIa antagonists and IABP. The next day she underwent resection of the small intestine due to superior mesenteric artery occlusion. Three weeks later she suffered from dyspnea, hypotension and VF, underwent a failed CPR and died. The second patient was a 79 years old post CABG man who suffered from amyloidosis. He underwent PCI and successful implantation of 2 Titan stents to a long lesion in the 1st marginal. Six weeks later he was hospitalised due to severe right heart failure, sinus bradycardia, paroxysmal atrial flutter and renal failure. He was scheduled for atrial flutter ablation and pace-maker implantation but deteriorated to severe dyspnea and desaturation and underwent a failed CPR.
Another 24 patients (8%) underwent angiography for chest pain and their coronary arteries stented with Titan were patent. At 180 days 83% of the patients had class 1 angina, 15% had class 2, 1% had class 3 and 1% had class 4 angina.
Discussion
The results of this multi-centre registry with the Titan, a Titanium-Nitride-Oxide coated stent are remarkably good. At 6 months there were only 5.7% TVR (of them 5.4% TLR), 0.7% MI and 0.7% death.
The inflammation burden and restenosis
In-stent restenosis occurs in 20-30% of patients undergoing stent implantation and is caused entirely by neointimal proliferation4,5. Inflammation is an imperative factor in the restenosis process. The inflammation burden prior and after angioplasty depends on the individual’s systemic and local (arterial) factors. Diabetes mellitus, vessel morphology (e.g. vessel diameter, lesion length, amount of residual plaque burden)6,7 and procedural factors (e.g. vessel wall trauma and basement membrane disruption) may all amplify in-stent restenosis.
The role of metallic implants in restenosis
Stent implantation adds iatrogenic factor by enhancing neo-intimal hyperplasia, a so called “response to foreign material”. Metallic implants can induce electron exchange (redox reaction), proton exchange (hydrolysis), and complex formation (metal ion-organic molecule binding) in living tissues8. The resultant protein (e.g. fibrinogen) activation, cell toxicity, fibroblast growth stimulation, platelet, monocyte/ macrophage and endothelial cells adhesion, and endothelial cell migration8 may all contribute to restenosis.
Stainless steel used to produce stents (316L) is made of iron fortified with carbon and contains significant amounts of Nickel (12%), Chromium (as chromate) (17%) and Molybdenum (2%). Ions of these elements elute from the stents and might induce the above mentioned reactions8. Nickel has been shown to cause skin contact allergic dermatitis in 5% of the male, and 15% of the female population9, and inflammatory and allergic reactions that form new tissue around metallic alloys containing nickel are well known in patients with dental and orthopaedic implants10. It is conceivable that stents which contain nickel and other elements would enhance neointimal hyperplasia formation. In a study performed by Koster et al, 131 patients were studied prior to angiography for suspected coronary in-stent restenosis of a stainless-steel stent9. All patients underwent allergy patch testing for nickel, chromate, molybdenum, manganese and stainless steel. Eighty-nine patients had restenosis and 10 of them (11%) had positive patch test results and underwent repeat angioplasty. Forty-two patients did not have restenosis and none of them had positive patch test (p=0.03). Thus, all 10 patients with positive patch test had in-stent restenosis. Another study failed to show correlation between nickel sensitivity and restenosis in a group of 20 post-angioplasty and 7 pre-angioplasty patients, but these groups were far too small to yield a measurable restenosis rate or draw any conclusion11.
Stent coatings
Several stent coatings such as heparin12,13, silicon carbide14 and gold15 have been constructed to create a biologically inert barrier between the stent surface and circulating blood but none of them decreased and some even increased15 restenosis rate. Although drug eluting stents (DES) decrease restenosis, they have not abolished it. An investigation of 8 different biodegradable and non-degradable polymers showed marked inflammatory reactions with subsequent neointimal hyperplasia16, and it has been proposed that this inflammatory process contributes to residual restenosis and possibly late thrombosis. Thus, there is still a need to improve bare metal stents features both as stand alone and platform for carrying neointimal hyperplasia inhibiting drugs.
The Titanium Nitride-Oxide stent
Titanium is biologically inert due to its low electrochemical surface potential. It has an excellent biocompatibility because it has neither redox or hydrolysis reaction nor complex formation and biologically it had no fibroblast growth, protein or platelet adhesion1,3,8,10. Titanium-nitride-oxide (TiNOX) is a titanium alloy suitable for coating of stainless steel stents preventing completely release of Nickel, Chromium, Molybdenum or other metals. In-vitro experiments have shown reduced platelet adhesion and fibrinogen binding to TiNOX coated stents during incubation in human plasma for 48 hours1. In-vivo experiments with the pig model have proved decreased cell and tissue reaction1.
In a recent small multi-centre randomised study, Windecker et al have shown reduction in angiographic and IVUS parameters of restenosis among patients treated with a non-commercial, custom made TiNOX stent compared to stainless steel stents, and this was translated into a lower MACE rate3. The lesions treated in that study were however undemanding and clinically complicated patients were excluded.
In the present study we used a commercial TiNOX stent (Hexacath, France). Experience with this stent has been presented recently. Karjalainen et al has performed a single-centre registry in Finland in 195 unselected patients (214 stents). At 9 months clinical follow up, MACE rate, cardiac death, MI and TLR were 10.3%, 0%, 4.1% and 5.1%, respectively17. Valdes et al have performed a multi-centre (9 hospitals) registry in Spain in 156 diabetic patients (197 stents). At 6 months clinical follow up, MACE rate, cardiac death, QMI and TLR were 10.3%, 1.95%, 1.29% and 7.09%, respectively. The authors considered these results to be the best ever encountered with bare-metal stents, and close to some drug eluting stents in diabetic patients18.
In this study we included elderly patients (68.8±11.8, 33-87 years old) with high risk profile; 36.6% were diabetics, 81% had ACS (36% hospitalised with acute MI) and 67% had multi-vessel disease. The lesions were mid or distal in >50% of cases, 62% were type B2 or C, and lesion length was 17.5±14.8 mm. Notwithstanding this highly complicated lesion and case mix, the results were excellent with only 5.4% TLR and 1.3% composite of death or MI. Post-mortem examination was not performed in the two patients who died and therefore stent thrombosis (non-Titan or Titan) can not be excluded. Non-coronary death is however more likely in the amyloid patient.
Possible contributors to these favourable results are TiNOX’s low electrochemical surface potential and the avoidance of nickel and chromium discharge. The role of NO attached to titanium is not known and has to be elucidated. Interestingly, pooled data of 15 Israeli national BMS registries (100 stents in each) showed 10.6% TVR and 1.3% death rate. In comparison, the Israeli Cypher registry had 2.2% TVR and 0.7% death rate at 6 months. Thus, the Titan results are situated in between other BMS and Cypher results. Also of interest, a recent meta-analysis of 3,669 patients treated with sirolimus- or paclitaxel-eluting stents, demonstrated TLR of 5.1% and 7.8%, death rate of 1.4% and 1.6%, and composite of death or MI of 4.9% and 5.8% within 6-13 months, respectively19. Since DES are not devoid of drawbacks including high cost, it is hoped that further developing of inert or bioactive stents such as the TiNOX might compete favourably with DES or serve as better platform for carrying neointimal hyperplasia inhibiting drugs.
Study limitations
Our study involves an inherent limitation of any registry, namely the non-blinded outcome assessment. Since not all hospitalised patients were treated with a Titan stent during the registry period, there might be a selection bias. This registry has a low/patient number experience. TLR was clinically driven and silent restenosis might have been overlooked. These limitations are partially counterbalanced by registries’ advantages over randomised trials; population selection and clinical relevance resemble real-life better, and unnecessary interventions in borderline lesions (that affect study results) are avoided.
In conclusion, the Titan is stent coated with titanium-Nitride-Oxide, which has an excellent biocompatibility and eliminates the discharge of elements such as nickel, chromium and molybdenum. This multi-centre registry demonstrated excellent short and long term outcome with very low clinical TLR, MI and death rates.