Abstract
Background: Even though sirolimus-eluting stents (SES) have been shown to significantly improve binary restenosis and target lesion revascularisation rates (TLR) compared to bare metal stents in diabetic patients, the revascularisation rate is still higher than those of non-diabetics. Whether a double dose (DD) of sirolimus on a stent would provide a greater reduction in neointimal hyperplasia compared to single dose (SD) SES in de novo coronary lesion of diabetic patients is unknown.
Methods and results: A total of 56 patients (58 lesions) were prospectively randomised in a double blind fashion in a 1:1 ratio to SD (30 lesions) versus DD (28 lesions). Procedure success was achieved in all patients. QCA results at 6 months were comparable between groups, including the primary endpoint of in-stent late lumen loss (0.19±0.29 mm in SD versus 0.18±0.33 mm in DD, p=0.96). Furthermore, restenosis was not found inside the stent in either group. By IVUS, there was no late/acquired incomplete stent apposition at follow-up, and% neointimal volume was 2.2±1.8% in SD versus 1.7±2.0% in DD, p=0.44. At 1-year clinical follow-up, there was no significant difference between groups for major events, including TLR which occurred in 1 patient in SD versus 3 patients in DD, p=0.61. Overall, there was only 1 subacute stent thrombosis (DD arm), and no late thromboses.
Conclusions: Double dose SES did not improve the prevention of neointimal proliferation in diabetic patients with de novo coronary lesion compare to single dose SES.
Diabetes mellitus remains one of the most important predictors of in-stent restenosis after percutaneous intervention,1-3 mainly related to the hyperproliferative vascular response.4
Sirolimus (rapamycin), a cytostatic macrocyclic lactone, is a potent immunosuppressive agent with antiproliferative and anti-inflammatory properties.5,6 Sirolimus-eluting stents (SES, Cordis Corporation, Miami Lakes, Florida) have shown sustained safety and effectiveness in preventing neointimal hyperplasia in coronary lesions, markedly reducing restenosis and repeat vessel revascularisation compared to bare metal stents.7-9 Even though SES have been shown to significantly improve outcome in diabetic patients, restenosis is still greater than in non-diabetics with a relatively high recurrence rate (up to 10.2% target lesion revascularisation).10-13
We sought to investigate whether a higher dose of sirolimus, when delivered from a polymer encapsulated stent, would provide additional benefits preventing neointimal proliferation (with sustained safety) in diabetic patients enrolled in “The randomised study of the Double Dose versus single dose SES for the treatment of Diabetic patients with De Novo coronary lesions” (3D) trial.
Methods
Study design
The “3D” study was a multicentre, prospective, feasibility randomised trial to assess the safety and effectiveness of double dose SES in diabetic patients with de novo coronary lesion compared to single dose SES. The study rationality was based on: (1) pre-clinical studies that demonstrated dose-related reduction in neointimal formation with SES; and (2) previous observations diabetics (especially insulin dependants) treated SES had increased neointimal proliferation compared to non-diabetics.11 Therefore, a “high” dose SES was considered to be tested in this subgroup. The drug dosage for the “double dose DES” was based on previous animal studies that showed absence of local vascular toxicity and systemic toxicity from exaggerated drug (and polymer) dose in pig coronary arteries. The maximum dose used in this pre-clinical study was similar to which a diabetic patient would be exposed to in this trial if they were implanted with overlapped 3.5 mm in diameter double dose SES. Patients were randomised in a 1:1 ratio to either the single dose – standard, commercially available SES (Cypher® stent) versus a study stent with a double dose of sirolimus coated onto the Bx-Velocity™ balloon expandable coronary stent (Cordis Corporation, Miami Lakes, Florida) platform. The SES coating consists of a blend of sirolimus and two non-erodable polymers. The standard SES contains 140 µg/cm2 of sirolimus whereas the double dose SES formulation used in this study had approximately 280 µg/cm2 of sirolimus. The copolymer matrix was designed to release 80% of the total sirolimus dose in 30 days. Stent assignment was blinded to both the physician operator and the patient.
Patients
Between June 2003 and January 2004, patients were enrolled at 3 clinical sites: Institute Dante Pazzanese of Cardiology, São Paulo, Brazil; Azienda Aspedaliera Ospedali Riuniti di Bergamo, Bergamo, Italy; and Lady Davis Carmel Medical Center, Haifa, Israel. Patients were eligible if they meet all of the following: at least 18 years of age; previously diagnosed with diabetes with documented treatment with insulin, oral medications, or diet for a minimum of 3 months; had diagnosis of angina pectoris as defined by Canadian Cardiovascular Society classification (CCS, I, II, III, IV) or unstable angina pectoris (Braunwald classification B&C, I-, II-, III-) or patients with documented silent ischaemia; acceptable candidate for coronary artery bypass graft surgery (CABG); presence of a de novo target lesion located in a native coronary vessel. Angiographic inclusion criteria included target lesion <30 mm in length in a vessel >2.5 mm and <3.5 mm in diameter (by visual estimation), with stenosis >50% and <100% (thrombolysis in myocardial infarction – TIMI flow >1). Exclusion criteria were pretreatment with devices other than balloon angioplasty; previous brachytherapy of target vessel; myocardial infarction – MI (Q wave or non-Q wave) with documented total cardiac enzyme (CK) level >2 times normal within the preceding 24 hours and the CK and isoenzymes CK-MB remaining above normal at the time of the procedure; left ventricle ejection fraction <30%; impaired renal function (creatinine >2.0 mg/dL); unprotected left main coronary disease with >50% diameter stenosis; significant (>50%) stenosis proximal or distal to the target lesion; target lesion involving a bifurcation with a side branch >2.5 mm in diameter; angiographic evidence of thrombus; total occlusion (TIMI 0); ostial lesion location; target lesion located in a saphenous vein graft; target lesion due to in-stent restenosis. All patients enrolled agreed to comply with the specified follow-up evaluation and provided written informed consent prior to the procedure using a form that was approved by the local Ethics Committee. Clinical follow-up was performed at 1, 6 and 12 months, and all patients were assigned for angiographic and intravascular ultrasound (IVUS) follow-up at 6 months.
Procedure
The randomisation was stratified by the type of diabetic treatment to ensure a balance between the two treatment arms of the trial. Predilation of the target lesion with an undersized balloon was mandatory. Stent were available in 8 mm and 23 mm length and 2.5 mm, 3.0 mm and 3.5 mm diameter. Multiple stenting was allowed to fully cover the lesion and/or for procedural complications such as dissections. Aspirin 325 mg and a 300 mg loading dose of clopidogrel or 500 mg loading dose of ticlopidine were administered pre-procedure unless patients were already pretreated. Heparin was administered to maintain an activated clotting time >250 seconds (>200 seconds if IIb/IIIa agents were used). Glycoprotein IIb/IIIa inhibitors were given per operator discretion, and all patients were prescribed aspirin (100-325 mg/day) indefinitely and clopidogrel (75 mg/day) or ticlopidine (250 mg bid) for at least 8 weeks. Post-procedure laboratory included cardiac enzymes (CK and CK-MB) within 6-8, 12-16, and 18-24 hours post-procedure, and an electrocardiogram was obtained within 24 hours post-procedure or at the time of hospital discharge.
Angiographic analysis
After intracoronary nitrate administration (50-200 µg), serial coronary angiography was obtained in multiple views at baseline, post-procedure and 6 months follow-up. Quantitative coronary angiography (QCA) was performed using the CMS-GFT® algorithm (MEDIS, Lieden, the Netherlands). All cine-angiograms were analysed at an independent core laboratory (Cardiovascular Research Foundation’s Angiographic Core Laboratory, New York, NY) blinded to the treatment protocol. The minimum lumen diameter (MLD) and the mean reference diameter (RD), obtained from averaging 5 mm segments proximal and distal to the target lesion location, were used to calculate the diameter stenosis [DS = (1 – MLD/RD) x 100]. Acute gain was the change in MLD from baseline to final post-stent implantation angiogram; late lumen loss was the change in MLD from the final post-stent implantation angiogram to follow-up. Binary restenosis was defined as >50% DS at follow-up, and was classified as focal (< 10 mm in length) or diffuse (<10 mm in length). All quantitative measurements were performed: (1) “in-stent” within the stented segment, (2) “in-lesion,” spanning the stented segment plus the 5 mm proximal and distal peri-stent areas, and (3) “peri-stent”, in the 5 mm proximal and distal areas immediately adjacent to the stent.
IVUS analysis
IVUS images were acquired by using automated pullback at 0.5 mm/s after intracoronary nitrates with one of two commercially available imaging systems (Boston Scientific, Natick, MA/Volcano Therapeutics, Rancho Cordoba, CA, USA). Two-dimensional and volumetric IVUS analysis was performed with the use of commercially available planimetry software (Tape Measure/Echo Plaque, Indec Systems, Mountain View, CA, USA), according to previously validated and published protocols. Vessel, stent, lumen and neointimal volumes were computed for the stented segment as well as stent margins 5mm distal and proximal to the stent. Percent neointimal volume (%NV) was defined as neointimal volume divided by stent volume multiplied by 100.
Endpoints and definitions
The primary endpoint was in-stent late lumen loss as measured by QCA at 6 months post-procedure. The secondary endpoints were evaluated at 30 days, 6 and 12 months post-procedure and consisted of composite of major adverse cardiac events (MACE) defined as death, MI (Q wave or non-Q wave), emergent CABG, or repeat target lesion revascularisation (TLR), TLR, target vessel revascularisation (TVR) and target vessel failure (TVF) defined as cardiac death, MI, or TVR. Angiographic secondary endpoints were assessed by QCA at 6 months post procedure and included in-stent and in-lesion binary restenosis, in-stent and in-lesion mean%DS and MLD, and in-lesion late lumen loss. Other secondary endpoints were glycemic control as measured by glycosylated haemoglobin (HbA1c), and C-reactive protein (CRP) levels, measured at baseline (within 7 days prior to procedure), 6 and 12 months related to patient outcomes. Device success was defined as achievement of a final residual DS <50% (by QCA), using the assigned device only. Lesion success was defined as the attainment of <50% residual stenosis (by QCA) using any percutaneous method. Procedure success was defined as achievement of a final DS <50% (by QCA) using any percutaneous method, without occurrence of MACE.
Statistical analysis
A sample size of 44 patients (22 for each group) would have 62% power (two group t-test with 0.05 one-sided significance level) to detect a difference in means of 0.20 mm in-stent late loss between the study groups, assuming a standard deviation of 0.33 mm. Data are presented as mean±standard deviation or frequencies. Statistical analyses were performed using the StatView 5.0 software (SAS Institute, Cary, North Carolina, USA). The treatment group differences were evaluated with the analysis of variance (ANOVA) or Wilcoxon Rank Sums Scores for the continuous variables. The Cochran-Mantel-Haenszel was used for categorical variables. The 95% confidence intervals were provided for the mean differences of QCA results and clinical events. The differences between the two survival curves were compared using the Wilcoxon as well as the log-rank tests. A p value <0.05 was considered significant.
Results
Single Dose (SD) versus Double Dose (DD) SES: baseline characteristics and procedural variables
A total of 56 patients were randomly assigned (SD=28; DD=28). Baseline clinical characteristics were comparable in both groups (Table 1).
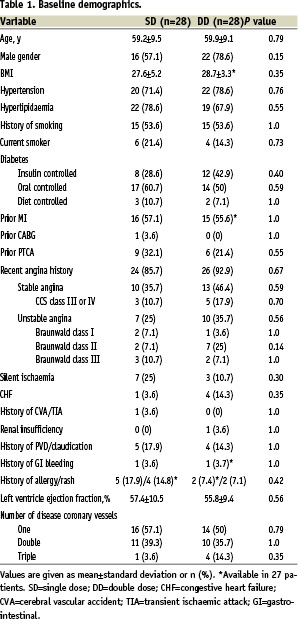
Mean age was 59.5 years, male gender was 67.9%, and 89.3% had a recent history of angina. Baseline HbA1C and CRP levels were 7.79% versus 7.94%, p=0.78; and 2.69mg/L versus 7.40 mg/L, p=0.09; for SD and DD, respectively. Among those with history of hyperlipidaemia (n=41), 92.7% were taking statins (p=0.78). Overall, 35.7% of patients were insulin dependent diabetics; the mean number of years since diabetes was diagnosed was 6.7±8.0 in SD versus 10.4±8.1 in DD, p=0.09.
Table 2 shows the preprocedure lesion morphology.
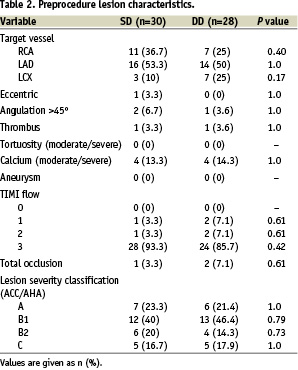
Two patients in SD had 2 lesions enrolled, therefore 58 lesions were analysed. LAD was the prevalent vessel in both groups and the majority of lesions (65.5%) were non-complex (types A/B1 according to the ACC/AHA classification). There were no ostial lesions, 13.8% had moderate to severe calcium and mean lesion length was 13.9 mm.
Procedural characteristics were comparable between SD and DD groups, including IIb/IIIa use (40.7% versus 48.1%, p=0.78), number of SES implanted per patient (1.25 versus 1.21, p=0.76), overlapping stents (25% versus 17.9%, p=0.75), and maximum stent deployment pressure (14.89 atm versus 14.52 atm, p=0.62), respectively; however, DD patients had more 2.5 mm stents implanted (23.5% versus 5.7%, p=0.045). All stents were implanted successfully, and final TIMI 3 flow was achieved in all but one lesion (one patient in DD arm had final TIMI 2 flow but presented with TIMI 3 flow at 6 months angiographic follow-up). There were no angiographic complications, such as dissections, abrupt closure or acute stent thrombosis, with 100% device, lesion and procedure success. At discharge, all patients were taking aspirin and thienopyridines, and 91.1% were prescribed to statins.
QCA and IVUS results
QCA results are shown in Table 3.
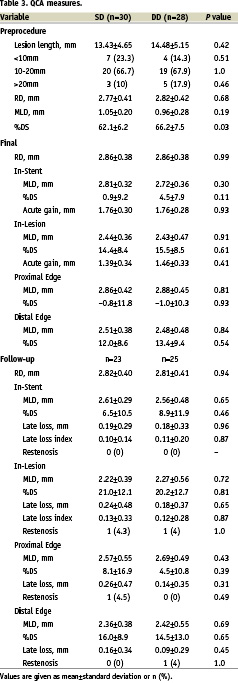
There were no significant differences between groups regarding vessel size and final angiographic measures. Angiography at 6 months was performed in 48 lesions (82.8%), and results were comparable between groups, including the primary endpoint of in-stent late lumen loss (0.19±0.29 mm in SD versus 0.18±0.33 mm in DD, p=0.96). Figure 1 shows the cumulative frequency distribution curves for in-stent MLD.
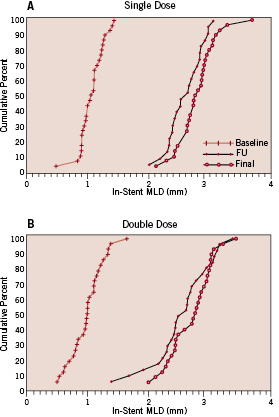
Figure 1. Cumulative frequency distribution curves for in-stent MLD in SD group (A) and DD group (B).
Furthermore, restenosis was not found inside the stent in either group; only one focal restenosis was found in each group – at the proximal edge in the SD group and at the distal edge in the DD group. Conversely, IVUS results showed no significant difference between SD and DD groups (Table 4).
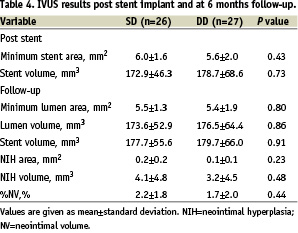
Incomplete apposition post stenting was found in 2 patients in SD and in 5 patients in DD. At 6 months follow-up, there was no late/acquired incomplete stent apposition. By QCA, aneurysm was not found in either group at follow-up.
Glycemic control and CRP levels
Glycemic control over the follow-up period, as measured by glycosylated haemoglobin levels, was stable and comparable in both groups. HbA1C levels for SD and DD at 6 months were 7.64% versus 7.83%, p=0.78; and 7.55% versus 8.30% at 1-year, p=0.30, respectively. CRP levels were also comparable, 2.46 mg/L versus 1.41 mg/L at 6 months p=0.43, and 2.04 mg/L versus 1.70 mg/L at 1-year, p=0.83, for SD and DD, respectively.
Clinical outcome
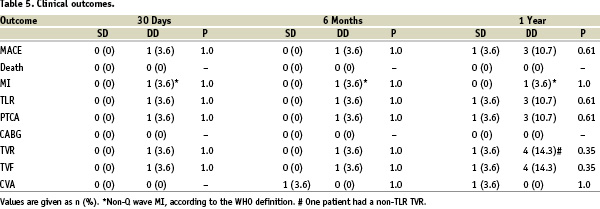
There were no in-hospital major events reported (0% MACE). Moreover, no statistically significant differences between SD and DD groups were observed regarding clinical outcomes over the follow-up period, including subacute stent thrombosis – 1 case in DD group. Except for 1 case of CVA in the SD group, there were no new major events between 30 days and 6 months. All patients completed 1-year follow-up and there were no deaths in either group. Figure 2 shows the 1-year event-free survival curves for TLR for SD and DD groups.
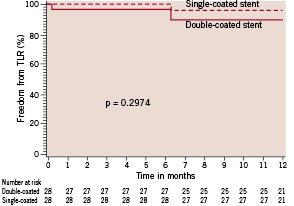
Figure 2. TLR-free survival curves in diabetic patients according to treatment assignment.
Furthermore, a total of 7 patients had non-TVR percutaneous intervention during follow-up, 6 of which presented between 6 and 12 months.
Discussion
The findings of this study of double dose SES in diabetic patients demonstrate: (1) a higher dose of sirolimus, when delivered from a polymer encapsulated stent, did not lower in-stent neointimal proliferation in diabetics compared to standard SES. In fact, both single and double dose SES demonstrated exceptional performance, considering this selected high risk subset (more than one third of patients were insulin treated), with extremely low late lumen loss and restenosis rates (0% in-stent restenosis) at 6 months follow-up; (2) SES effectively prevented neointimal hyperplasia (0.18-0.19 mm in-stent late lumen loss by QCA, 1.7-2.2%NV by IVUS), but did not prevent progression of disease outside the stent. Actually, the majority of vessel revascularisation, including the TLRs, occurred between 6 and 12 months follow-up. This confirms the aggressive nature of the atherosclerosis progression in diabetics.14-16
In-stent late loss, a surrogate of neointimal hyperplasia, was virtually the same in both treatment arms. There were similar biological responses despite significant different overall amounts of drug (71-221 µg of sirolimus in single dose versus 142-442 µg of sirolimus in double dose), but similar release kinetics. In a porcine model,17 sirolimus produced a relatively constant degree of inhibition of neointimal hyperplasia over a 200-fold dose range from 6 to 1,200 µg. The finding of this clinical trial provides support to the preclinical results that sirolimus has wide therapeutic window. The performance of the single dose SES resulted in late loss lower than expected in this trial (0.19 mm), virtually abolishing neointimal tissue (2.2%NV); therefore, the expected difference in in-stent late loss between both groups used for sample size calculation was just not seen.
In-stent restenosis was 0% in both single and double dose groups. Overall TLR was 7.1%. During the late clinical follow-up, three patients had TLR (two in the double dose group and one in the single dose group). Two TLR were related to progression of the disease outside a patent stent (one in each group), and one had re-obstruction of the stent (double dose group). The discrepancy between in-stent (minimal progression of the disease) versus in-lesion (progression of the disease associated with TLR) highlights the importance of complete lesion coverage, especially in diabetics. In SIRIUS, insulin dependence significantly impacted TLR (64% increase compared to non-insulin controlled).11 In 3D, only one TLR was related to an insulin dependent patient.
Previous studies have suggested that polymers are an essential component of DES.18 In the DELIVER trial, paclitaxel-eluting stents without polymer demonstrated disappointing results, basically by the lack of controlled drug release.19 However, polymers have been associated with an intensive inflammatory response (especially against durable polymers).20-22 SES uses a non-erodable polymer as a drug carrier.11 In our study, sirolimus was eluted in 209-657 µg of polymer in SD versus 291-908 µg in DD. Interestingly, this difference appeared to have no impact on the degree of inflammatory response observed. Neointimal tissue formation, measured by QCA (late loss) and IVUS (NIH and%NV) at 6 months was considerably low, with similar results in both groups. Also, CPR levels were comparable at 6 and 12 months. The fact that the polymer was proportionally blended with sirolimus (a potent immunosuppressive agent with anti-inflammatory and antiproliferative properties)5,6 may have restrained an exaggerated local inflammatory response. This has been demonstrated in animal studies.21 Furthermore, there were no aneurysm formation in either groups, and 4 of the 5 stent incomplete apposition found post-procedure in the double dose group were either preserved (no worsening, n=3) or healed (n=1) at follow-up (one patient with incomplete apposition did not undergo IVUS follow-up). Nevertheless, a recent study with a new generation polymer-free SES demonstrated promising results in reducing restenosis.23
The subacute stent thrombosis rate in the double dose group was 3.6%, but this corresponded to only one patient who deployed two long overlapping stents (46 mm) in the LAD with diffuse disease distally. The patient returned 3 days later with total occlusion of the vessel. There was no subacute thrombosis in the single dose group. Diabetic patients treated with Cypher® stent in the SIRIUS trial were also reported to have 0% subacute thrombosis.11 Importantly, in our study neither group presented with late thrombosis. The small sample of this study precludes definite conclusions about the implications of the safety of the double dose device. A larger study is needed to fully investigate the safety profile of the double dose SES.
Conclusions
The double dose SES did not improve the effectiveness of the single dose SES in reducing neointimal proliferation in diabetic patients. Both single and double dose SES were associated with a high procedure success rate and low incidence of events, showing a wide therapeutic window for safety and effectiveness in reducing neointimal hyperplasia in a high risk subset of diabetic patients.
Acknowledgments
An institutional grant was given by Cordis, a Johnson & Johnson Company, Miami Lakes, Fla, USA. We thank Dr. Dennis Donohoe for his careful review of the manuscript.

