Introduction
Stent implantation during coronary intervention has been shown to be very beneficial to maintain blood flow through the arteries after treating coronary stenosis. In bare metal stents, after a period of six months neointimal hyperplasia growth compromises the lumen in 26% of the cases, requiring repeated catheterisation1. With the advent of drug eluting stents, restenosis rates are decreased tremendously on the account of a very small percentage late thrombosis risk (2.6%)1. Rheologic factors has been shown to be involved in numerous processes, including neointimal growth and thrombus formation. The formation of neointimal hyperplasia in stented arteries has been linked to persistence of low shear stress as is platelet adhesion. The role of shear stress in in-stent thrombus formation received much less attention.
In this review we will discuss state-of-the-art research on the involvement of shear stress in neointimal growth in bare metal and drug eluting stents and highlight a number of studies on thrombus formation.
Shear stress and vascular (patho)biology
Haemodynamic forces acting on the vessel wall
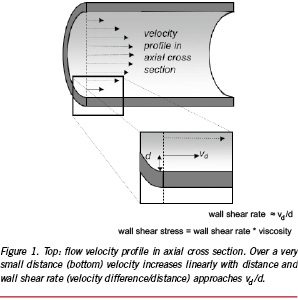
As blood flows over the endothelium, it exerts a frictional force on the endothelium. Although the force of the flowing blood is very small compared to the forces induced by the blood pressure, the endothelium is very sensitive to it. The frictional force acting at the wall induced by the blood flow is often expressed as the wall shear stress, which is the drag force acting on the luminal wall normalised to wall area. In a simple straight tube the Hagen-Poiseuille formula (Shear stress = 4µQ/πR3, with µ viscosity, R tube radius and Q flow) can be applied for steady laminar viscous flow. Applying that simple equation, an average normal shear stress of 0.68±0.2 Pa was derived from Doppler based velocity measurements in angiographically normal coronary arteries of 21 patients2. The endothelium responds to the shear stress by aligning to the flow direction, gene expression and production of many anti-atherogenic substances, including NO and endothelin3. In contrast, low and oscillatory shear stress regions are a pro-atherogenic environment and in the presence of risk factors atherosclerotic plaques are prone to develop3.
Shear stress and neointimal hyperplasia
Neointimal hyperplasia after percutaneous transluminal coronary intervention results mainly from vascular smooth muscle migration, proliferation and matrix formation. Three distinct phenomena lead to neointima hyperplasia: (1) The expansion of the stent wires at the time of implantation can result in severe vascular trauma (“overstretch injury”) which has been quantitatively related to the extent of neointima hyperplasia4. (2) Stent implantation induces complex interactions between blood and vessel wall components, and the metal structure of the prosthesis. The materials and the roughness of stent surface can affect the absorption of plasma proteins which generates an inflammatory response5. (3) Stent implantation alters the coronary flow and geometry and thereby induces changes in local shear stress distribution.
Mechanisms by which shear stress may modulate the vascular smooth muscle cells (VSMC) proliferative and migratory responses in vivo include induction of mitogenic cytokines, such as PDGF and matrix metalloproteinases6. Since the integrity of the endothelial layer is damaged directly after balloon angioplasty or direct stenting other pathways than the endothelium might get activated. Sterpetti et al reported7 that VSMCs also sense shear stress shear stress such that high shear stress directly inhibits VSMC proliferation. Recent studies indicate that when cultured VSMCs are in the synthetic phenotype, they respond to shear stress in an analogous manner to the endothelium, altering their production of growth factors8. In the balloon catheter-injured and de-endothelialised rat carotid artery, the VSMCs that migrate through the internal elastic lamina to form the neointima rapidly change to the synthetic phenotype, and they maintain phenotypic modulation until at least two weeks after injury7. Thus, it is possible that juxtaluminal synthetic VSMCs could respond to abnormal shear forces in a manner similar to the endothelium. As the VSMC play an important role at the short-term, at the long-term, the endothelium is regenerated and could respond to the local shear stress field by producing growth factors; anti-thrombotic and anti-inflammatory agents. So both at the short-term and long-term shear stress potentially controls neointimal growth.
Shear stress and thrombosis
Blood rheology has impact on many processes involved in thrombus formation, including platelet recruitment to the vessel wall, platelet adhesion, activation, and aggregation9. The redistribution of blood cells is one of the important effects of blood flow. This is particularly important as erythrocytes are transported to the central part of the tube leaving the platelets close to the vessel wall10. As the platelets are closer to the vessel wall, they can more easily adhere to the vessel wall than if the erythrocytes were not transported to the main stream. On top of that plasma proteins, involved in platelet recruitment and activation, might be trapped in recirculation zones leading to concentration increase of such proteins close to the vessel wall11. Rheological parameters have also a direct effect on platelet adhesion. Under low-flow rates platelets can interact and stably adhere to receptors expressed at the endothelial surface such as αIIβ3.12 At high shear rates, the platelet receptor GPIb-V-IX complex via its association with vWF can decelerate the platelets in the flowing blood and thereby stimulate binding to other platelet receptors13. Platelet activation is mainly driven by high shear stress (15-60 Pa) found in, for instance, stenosed atherosclerotic arteries14. Activated platelets release many growth factors, including PDGF, basic fibroblast growth factor, and transforming growth factor. These factors play an important role in regulating VSMC migration and proliferation15. Bassiouny et al found6 that platelet activation after experimental arterial injury as measured by thromboxane B2 levels was greater with injured arteries subjected to reduced flow compared with normal and increased flow conditions. This suggests that platelet adhesion to regions of injury is enhanced in a low-flow and low-shear environment.
Shear stress calculations and restenosis in patients
Computational fluid dynamics
To study the involvement of shear stress in in-stent restenosis in patients requires assessment of the local shear stress and thus the velocity gradient close to the vessel wall. Local measurements of the velocity gradient in human coronary arteries in vivo is very difficult and can therefore not be applied to map the shear stress distribution at the wall. An alternative way to determine the local wall shear stress in these arteries is application of computational fluid dynamics16 in 3D reconstructions of coronary arteries. 3D reconstructions of human coronary arteries can be obtained using biplane angiographic images, but provide elliptical cross sections only and therefore has limited applicability17. To overcome this limitation, we developed the ANGUS method offering an attractive alternative for 3D coronary artery reconstruction; this method combines biplane angiography with high-resolution IVUS imaging permitting to include 3D-reconstruct the arterial wall18 (Figure 2). For the computations, as a first step, a computational grid fitting in a 3D reconstructed lumen of human coronary arteries is generated (Figure 3A). Measured flow rate and patient specific blood viscosity data are used for the boundary conditions and material properties. The computational grid and the input conditions are used to solve the Navier-Stokes equations by means of computational fluid dynamics. This procedure renders the local velocity (Figure 3B) and shear stress distribution at any point in the computational grid of the reconstructed human coronary artery (Figure 3C and D). This is of great value, as it allows in vivo studies relating spatial wall characteristics with haemodynamic parameters derived from computational fluid dynamics applied to the lumen reconstruction16,19-23.
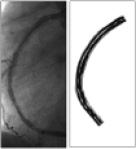
Figure 2: 3D reconstruction of human coronary artery: A) angiogram and B) ANGUS 3D reconstruction.
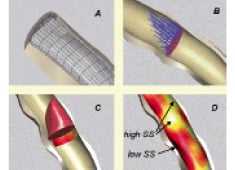
Figure 3. (A) After lumen meshing, computational flow dynamics allows (B) detailed velocity determination at any cross section. From this (C) the local velocity profile and wall shear stress (coloured band) are derived. Ultimately, (D) shear stress is calculated at any location of the lumen wall as shown in colour code. Note that in general, at the outer curve of this human coronary example, WT is smallest and shear stress highest, while the reverse can be noticed at the inner vessel curve.
Influence of stent design on shear stress distribution
A number of groups studied the influence of stent presence and design on the changes in shear stress profiling19,24-31. We showed in five healthy coronary arteries of pigs that stent implantation increased the curvature at the entrance and exit of the stent, resulting in shear stress maxima and minima19. Similar results were found in human coronary arteries by LaDisa et al, who demonstrated that the low shear stress areas present extended to the distal side of the stent. Inside the stent the minimal shear stress decreased by 77%26. They, however, also included the individual stent struts in their simulations. Based on a theoretical model of an implanted stent, the influence of the size, the number and the expansion ratio of the stent struts on the local shear stress was investigated26. Increasing strut thickness (0.056 to 0.096 mm) and number of stent struts (four to eight) resulted in an increase of 570% and 175% respectively, in area exposed to low shear stress. In contrast, Dubini’s group showed that strut thickness increase in stents expanded in a more realistic geometry induces a reduction in low shear stress area in the deceleration phase of the cardiac cycle only27. So stent design might contribute to risk on restenosis and thrombosis because of its influence on shear stress.
Influence of shear stress on the development of neointimal hyperplasia
Among other localising factors for neointimal hyperplasia formation, including plaque burden32 and wall (tensile) stress33, shear stress has been increasingly recognised as important modulating factor for tissue proliferation34. We studied the role of shear stress in 14 coronary arteries of patients six months after Wallstent implantation20. The average relationship between neointimal thickness (NIT) and shear stress (WSS) was: NIT=(0.59±0.24) –(0.08±0.10)⋅WSS mm, (p<0.05). These data imply that neointimal growth is located at the low shear stress regions showing resemblance with the results of Garcia et al., who studied in-stent neointimal growth in seven patients using similar techniques35. This was also true in a number of animal studies in which the localisation of in-stent neointimal formation and hyperplasia in bypass grafts versus shear stress was investigated36-38. Vice versa, if the shear stress was artificially enhanced, neointimal formation was reduced compared to the control situation34,39. In one study, the shear stress was enhanced by placing an additional device (a flow divider) in the centre of the stent, such that the resistance was minimally influenced, while the shear stress was maximally increased (Figure 4). These stents, including flow divider, were placed in the femoral arteries of atherosclerotic rabbits, which resulted in a doubling of the shear stress and a reduction of in-stent neointima hyperplasia by >40%. In contrast, Stone et al did not find differences in (n=6) neointimal growth for the different shear stress regions within a stent40.
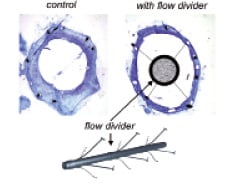
Figure 4. Cross-sectional histologic specimens of iliac arteries of rabbits after placement of a flow divider in the centre of a stent that increases shear stress at the stent surface. This resulted in a reduced intimal hyperplasia as described in the text.
Studies in drug eluting stent
Drug eluting stents (DES) represent the current most effective tool for the prevention of restenosis after PCI41. The question is whether neointimal growth in the presence of sirolimus/ paclitaxel still responds to shear stress as described above. Only a few papers studied this issue with opposing results.
We studied the relationship between shear stress and neointimal growth six months after implantation of sirolimus eluting stents in human coronary arteries. The average neointimal growth was – 0.03±0.03 mm, implying that shallow pits because of tissue regression were observed between the stent struts. Combining the data of all patients, a significant inverse correlation was found between neointimal growth and shear stress: NIT=0.21 mm±0.24* shear stress (r2=0.24, p<0.001) (See figure 5). In this study, the negative relationship between neointimal growth and shear stress meant that deeper pits between the stent struts were present in the high shear stress regions along the outside curve of arterial segments. Unlike our results, in diabetic patients (n=20) no relationship between neointimal growth and shear stress was observed nine months after implantation of sirolimus eluting stents42. In contrast, in a porcine coronary artery model in which sirolimus eluting stents were implanted, an average neointimal growth of 2.50±0.47 mm2 was observed 90 days after implantation43. Furthermore, in those stents, a positive relationship between neointimal growth and shear stress was found. As they assessed the shear stress based on the “Poisseuille formula”, assuming straight pipes, no shear stress variations because of stent curvature were taken into consideration which could have influenced their results.
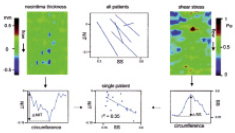
Figure 5. Relationship between neointimal thickness in sirolimus eluting stents and shear stress.
Papafaklis who studied a single patient treated with a paclitaxel eluting stent, showed that both neointimal growth and tissue regression were present, but that only neointimal growth was inversely related to shear stress. They found no significant association between tissue regression depth and shear stress44. Post-procedural stent malapposition in combination with inhibition of VSMC proliferation and clearance of post-procedure thrombotic material or apoptotic cells from the damaged vessel wall thrombus dissolution are potential mechanisms for the lumen enlargement in this case. In addition, a temporary sirolimus-stimulated production of prostacyclin45, which is a potent vasodilator, may also have played a role.
Shear stress and in-stent thrombosis
Stents are known to be thrombogenic46 and it is reasonable to suspect that flow disturbances because of stent implantation stimulate thrombus formation. Although a number of studies showed the influence of the haemodynamic consequences of lumen narrowing on thrombosis formation and thromboembolisms, research on the influence of shear stress on in-stent thrombus formation is in its infancy.
In a stented arterial model, the spatial distribution of platelet deposition close to stent struts was studied under physiological flow conditions47. In this model, stent struts were positioned on a parallel plate and the influence of the ratio between inter-strut spacing and struts thickness on platelet distribution was studied. In regions distal to the stent struts, the velocity lines were directed towards the wall and that resulted in the greatest platelet deposition. However in regions were the streamlines were directed away from the wall, this is at reattachment points, recirculation proximal to the struts, corner flow regions, platelets were less de-positioned.
Sukavaneshvar et al48 studied the influence of residual stenosis proximal and/or distal from a stent on both thrombus and emboli formation in an experimental set-up. In this experimental set-up, a Ni-Ti alloy coronary stent with five V-shaped struts (strut size =2.5 mm) was deployed inside the PVC tubing using a conventional polyethylene percutaneous transluminal balloon angioplasty catheter. They showed that a residual stenosis upstream and downstream lead to increased thrombus formation. On the other hand, emboli were more frequently detected if a stenosis was present either at the upstream or downstream side of the stent. Indeed, in a human coronary artery in vivo, a residual stenosis because of undersizing of the stent led to thrombus formation, presumable caused by abnormal shear stress patterns49.
Conclusion and clinical implication
Shear stress is of paramount importance in vascular biology since it has multiple effects on endothelial and vascular smooth muscle cells. With advanced technologies it became feasible to investigate these mechanisms and their effect after vascular intervention. It was demonstrated that low and oscillating shear stress can be associated with increased in-stent restenosis and thrombosis rates.
On a macroscopic scale, stent placement induces straightening of arterial segments, thereby causing a change in the shear stress distribution. On a microscopic scale, height of the stent struts and design pattern strongly influence shear stress. It was previously shown that by changing stent design shear stress in the stented segment could be raised and that this increase in shear stress significantly reduced neointima formation. Combining the expertise of clinicians and engineers might enable us to further improve the design of stents such that the resulting shear stress distribution minimises restenosis and thrombosis rates.

