Despite our best intentions to live according to carefully planned goals, life has a tendency to express itself in curious ways and unexpected outcomes. Now nearing the conclusion of my medical career I come to realise that serendipity had as much intervened in my life as anything I had under my control. If I could identify the things
I have done right without trying, and pass that experience on to young people, then I would be smart as opposed to just lucky.
I came to the US from my native Argentina when I was half my present age, with the firm determination to do something significant in vascular research. However, this goal was as broad as the subject itself, and looking for opportunities was a humbling experience.
The opportunity came all of sudden while I was attending my very first vascular meeting, as a first year resident. The keynote speaker of the February 1978 meeting of the Society of Cardiovascular and Interventional Radiology (SCVIR) was scheduled last in the program, not because of the importance of his subject but as a perverse way to keep the audience from dwindling during the last session on a late Friday afternoon. The speaker was none other than Andreas Gruentzig in one of his first presentations in the US of a fascinating new way to treat vascular disease, balloon angioplasty. I was seated in the first row of the crowded, small room so I had a good look at the young man, who was dynamic and engaging. Gruentzig came across as both brilliant and honest as he showed his early experiences promising, but not free of pitfalls. In fact, he spent as much time talking about failures as he did about successes. He showed several angiograms and histological sections of the new evil resulting from the brand new technique. Acute post-angioplasty dissection and occlusion were the unpredictable complications arising after deflation and withdrawal of the angioplasty balloon. My mind has always seemed to seek a solution whenever a problem is presented to it. The faltering arterial wall after balloon angioplasty elicited for me the image of crumbling soil in oil drilling, requiring the placement of a metal tube casing. On the way to the New Orleans airport in the front seat of the cab with my boss Stewart Reuter, I commented on Gruentzig’s new technique and a potential solution to its limitations. Stewart, who was the president of the SCVIR that year, thought that I ought to write up the idea as a way to exercise my medical writing abilities. The report I produced, after some bibliographic research and work at home, constituted the written evidence of the first conception of the idea, and would prove an important document in court battles 20 years later.
A garage was the birthplace of the balloon-expandable stent
The balloon-expandable stent evolved while I played with the idea working on a work bench in my garage with wire, wood dowels and pins. The pins stuck on the dowels served as guides for the wire to form a mesh. The mesh evolved from overlapping to interwoven wires and eventually the wire cross-points were soldered. The materials were procured from the local Radio Shack store, and used balloons and catheters from the hospital completed the materials for the crude prototypes. Rubber tubes affixed to plywood board made for the early test jigs. The stents were made of copper wire soldered with lead, unacceptable materials for implantation, but easy to use. Later, I replaced them by stainless steel suture wire and silver solder. The use of dissimilar materials in the construction of the stent bothered me, because in my readings of bio-materials, this was less than ideal. The answer to that problem had been staring me in the face for quite a while - this was a piece of expanded metal, left by
a carpenter after building a closet for the washing machine. The staggered diamond mesh was similar to my woven wire mesh after rolling the material into a tube. By pounding the material in the bench vise and forcing the mesh closed, staggered diamonds became staggered slots. That was the solution.... Staggered, parallel slots etched on the wall of a solid tube. Made in the right thickness and configuration, the tube in its unexpanded state, could be mounted on a folded angioplasty balloon. The single piece tubular construction had the advantage of decreased wall thickness as overlapping wires were avoided. Upon inflation, the balloon would expand the stent to a larger diameter while dilating the vessel simultaneously (Figure 1). Unlike the woven design, I had no way to make a slotted tube, so I resorted to making large scale models out of thin cardboard for these early experiences with mesh configuration, open area and shortening and dilating ratios. I tested radial strength in function of wall thickness and mesh density in woven stents, in jigs made of PVC pipe and rubber glove fingers.
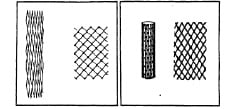
Figure 1. Illustration of the balloon-expandable stent in the unexpanded and expanded state. The left drawing shows the woven wire stent with soldered cross points. The right drawing shows the single piece, slotted tube stent. J Palmaz. original monograph, 1980.
My understanding of the working principle behind the balloon expandable stent came from cutting woven stents in progressively smaller pieces with scissors. As long as there was one continuous ring of connected struts around the circumference, the stent opposed a radially applied force. Therefore, the lack of longitudinal flexibility of the slotted tube, could be avoided by placing smaller units of the mesh in tandem, linked together or not.
I had read that metal devices have been implanted before in bodily tubes such as bile ducts, ureters and urethra with poor results. Metal in direct contact with mucosa in an ionic fluid medium invariably causes proliferation, slough and inflammatory changes leading to certain occlusion. This was a much feared outcome that had to be ruled out in vascular stents. However, a different type of response to metals could be anticipated in the intravascular space, based on the known healing response to prosthetic aortic valves and intracaval filters.
I considered at that point, that contacting a company interested in developing the device was the next logical step. This was a waste of time. Almost two years went by, while two companies in succession examined the project and finally decided not to get involved. A representative of a third company rejected the idea over the phone but suggested the name of a person who could help. This was Werner Shultz, who lived in Willits, California, a three hour car ride north of the Bay Area, and who was a retired biotechnologist from Stanford University. After examining my drawings and cardboard models Werner told me about several alternative ways to make the device, the most practical being electromechanical discharge machining or EDM. Making working prototypes of the stent was beyond his interest and capabilities, but he accepted to remain in contact for future advice. I went back home with renewed enthusiasm and revised my monograph, adding a section on the manufacturing methods as suggested by Werner. To my dismay, Mr Schultz died suddenly a few weeks later. His death precipitated my decision to move out of the Bay Area, to a place where I could devote undivided attention to the stent project. The University of Texas Health Science Centre in San Antonio offered me substantial research time and facilities, as well as happening to have an EDM machine on campus. Therefore in 1983, I accepted a position and stayed there for the next 22 years.
Going to Texas
Even before arriving in San Antonio in October 1983, I had my work organised and my research catheterisation laboratory ready. With no time to waste, I made some woven stents and tried them for the first time in laboratory animals. Everything worked just fine. Despite the fact that the prototypes were crude and bulky, the promise of the technique was evident. Positioning of a balloon-expandable stent under fluoroscopy was very simple, and deployment of the device by balloon inflation gave a definite sense of control. The superior mechanical advantage of the balloon expandable stent was evident in human cadaver arteries with atherosclerotic disease. The radially applied force displaced eccentric plaques so effectively that the cross section of the arteries became oval in shape (Figure 2). We further tested the mechanical advantage of balloon deployment by blocking blood flow in the abdominal aorta of a dog with a rubber band and reopening it with a stent. However, the real joy of discovery came by examining the first implanted specimens. With Dr. Fermin Tio, a vascular pathologist at the University, we observed that the metal devices initially covered by a layer of fibrin, became endothelialised in a very short period of time1. Dozens of questions rushed to my mind as I realised I knew almost nothing about intravascular stents. Would stents heal similarly in vessels of small diameter? What would be the smallest vessel that could be stented? Would a stent heal as favourably in a diseased vessel? How would a stent heal in the growing vessel of
a baby?
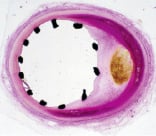
Figure 2. Cross section of a human cadaver coronary artery. An expanded stent forces an eccentric plaque outwardly, forcing the artery to become oval. Methacrylate, H&E. 40X
With lots of projects in mind, but with little money to finance them, my next problem was to obtain research funds. The university was generous in lending me facilities and seed money to get started, but it was time to get serious grants. Things were not going well in this respect. A grant for a coronary stent project application was rejected by the VA Research office, and the Patent Committee of the UTHSCSA deemed the balloon-expandable stent not worthy of a patent application. Looking for help at the Southwest Research Foundation, I met another under-funded investigator named Richard Schatz. Richard loved the stent at first sight and offered to help. A few days later he called me with the news that a restaurant entrepreneur, Phillip Romano, was interested in investing money in the stent project. I was confused because I didn’t know how to deal with a venture capitalist. Nonetheless, Richard, Phillip, Phillip’s young lawyer and I met shortly after. After I made my brief presentation I made sure Phillip understood that the stent was a project which had been rejected by several companies, the federal government and the University of Texas, hence it would clearly be a bad investment opportunity. Only the lawyer agreed with me as Phillip grew more interested, the more pessimistic my comments became. At ease with my conscience, I proceeded to ask for four times what I had been seeking elsewhere and Philip agreed. His only condition was that a company should be formed with the purpose of developing the balloon-expandable stent. After a month of due diligence, Phillip, Richard and myself formed Expandable Grafts Partnership or EGP.
Flushed with fresh funds in my research account, I asked my boss to switch my duties to 100% research time. This was agreed on, as long as I took care of my on-call duties. Immediately, I hired help and expanded my research lab. In short time, several new projects were started, exploring my earlier questions, while posing some new ones.
The animal catheterisation lab became quite busy and many projects got under way including1: Stents in an atherosclerosis rabbit model2; Stents in normal and stenotic renal arteries3; The effect of antithrombotic medication on stents4; The use of stents as intrahepatic porto-caval shunts5; The effect of re-expanding stents in growing vessels6. However, the most important project was the trial of stents in coronary arteries, as this project anticipated many of things
to come, such as acceptable patency rates and the decrease in neointimal thickness with time7. Two repeated failures to maintain patency of coronary stents in baboons were followed by a string
of successes in the coronary arteries of dogs, after we had tuned up the procedural anticoagulation (Figure 3).
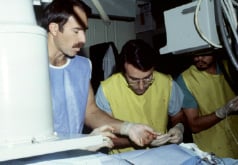
Figure 3. Richard Schatz (left) and Julio Palmaz (right) working in the UTHSCSA research laboratory. San Antonio, 1985.
With a more substantial package of evidence compared to two years before, Richard, Phillip and I started looking for a company to sponsor the project. In the mean time, I prepared an application for an Investigational Device Exemption (IDE) to the FDA on a preliminary trial of stents in atherosclerotic occlusion of human iliac arteries. Despite the fresh evidence that the stent was working well in laboratory animals, the companies we visited were still not interested in any substantial manner. All this changed when we showed the project
to Johnson and Johnson. As part of their due diligence, they hired a consulting company to assess if the stent project would be befitting of a company of the magnitude and prestige of J&J. Despite the consultant’s recommendation against getting involved with the stent, J&J licensed the project in 1986 from EGP, and this started a long-term relationship with the giant medical company.
Johnson and Johnson initially assigned the stent project to Ethicon, Inc, one of their subsidiaries, because of their involvement in implantable materials such as surgical sutures and staples. However, shortly after a tiny stand alone company was formed, Johnson and Johnson Interventional Systems or JJIS. The seven employee group got busy with several tasks: firstly, they recalled my IDE application from the FDA, modified it, and sent it back under their sponsorship and secondly, the seminal division between peripheral and coronary stents started on the engineer’s drawing board as the first peripheral stent was designed for specific use in the iliac trial. The first coronary stent design had to address the issue of lack of longitudinal flexibility if substantial lengths were to be used. This limitation was evident in our first trials in dog coronaries and Richard and I had been looking for solutions. In trying to adapt the straight, rigid stent for use in the coronary arteries Richard was a proponent of a simple approach; Use two short straight segments joined in the middle by a simple bridge (Figure 4). I hated the idea because of the gap between segments, but agreed that such a stent would be easy to make.

Figure 4. Palmaz Stent (top) and Palmaz-Schatz stent (bottom).
In 1985 while EDM was widely available, laser cutting techniques were not yet in widespread use. EDM however, was limited to straight cuts and this limited the design of the stent to straight features. Richard and I regarded the Palmaz-Schatz stent obsolete before it went into use. Even before it did, we conceived of more elaborate designs (Figure 5), but they would require time-consuming and expensive fabrication methods. Johnson and Johnson opted for the simple alternative as the first practical iteration.

Figure 5. Palmaz-Schatz stent with spiral connectors.
A change in life style
All of the sudden the research laboratory was not my usual dwelling place any more. The need to train people in the use of the stent in peripheral vessels took me to far and diverse places. Richard started doing the same with the coronary stent. We both began to spend more time abroad than at home, and became premium frequent flyers.
The first balloon-expandable stent placement in a human iliac artery was in May 1987 at the University of Freiburg, (then West Germany) with Goetz Richter and Professor Wenz. The patient was a pleasant 64 year old man with a totally occluded left common iliac artery8. To get to the point of actually intervening took three trips to Freiburg and many hours of meetings, conferences and interviews to get acquainted with members of the departments involved in that first trial. At that time, German hospitals had the freedom to perform first-in-human trials without supervision from outside agencies. However, the scrutiny over the risks and benefits to the patient were as stringent as I have ever encountered. The first stent procedure was flawless, and the patient’s lower extremity ischaemia resolved. Unfortunately he died two years later of cancer, but his family, by agreeing to an autopsy, provided invaluable evidence as to the long-term healing of the stent in an atherosclerotic vessel. Just as we had seen in animals, the stent embedded in fibrocollagenous tissue
displaced and isolated a complex plaque while restoring blood flow (Figure 6a, 6b).
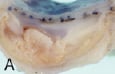
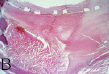
Figure 6a. Gross specimen cross-section of a common iliac artery, 26 months after stent placement. 6b. Histological section corresponding to fig 5a. H&E 40X.
In December of the same year Richard and I flew to San Paulo, Brazil for the first coronary stent placement with Eduardo Souza. The patient had a totally occluded RCA and became the first and longest bearer of a balloon-expandable coronary stent until he ironically died in an automobile accident after 17 years in good health.
Barry Katzen, Gerald Dorros and Ted Dietrich were among the early investigators for iliac and renal stent trials, which showed promising results9,10. I served as the principal investigator in the iliac stent trial, and then presented the experience before the FDA panel. The iliac stent got an approval in the US in 1991. It was the first vascular stent approved for human use by the FDA. By this time, significant clinical experience had already been gathered with the coronary stent in Rotterdam, Kokura, Mainz, Nancy, Milan, Madrid, Buenos Aires, as well as other places.
As it happens with all large trials, some remarkable patients were recruited. One of the most notable individuals in the coronary stent series was Mother Teresa of Calcutta, India. Mother Teresa urgently received a Palmaz-Schatz coronary stent at the Scripps Clinic in La Jolla, California in December 1991 for an acute coronary syndrome. Surrounded by lots of publicity, Mother Teresa made a startling recovery and departed the Scripps Clinic leaving many people mesmerised with her personality.
People at JJIS thought that the collective experience accumulated thus far was plenty to justify an application for FDA approval. They were wrong. In December 1993 the first presentation of the coronary series at the FDA by Marty Leon, Shelly Goldberg and Don Baim was rejected. The FDA panel presided by Eric Topol could not agree to approve without a randomisation study. This was disappointing because that year two different types of laser as well as
a rotational atherectomy device were approved without randomised data. The rejection was a hard blow to the project, but despite the disappointment things moved forward thanks to the determination of Marvin Woodall – head of JJIS – and his team. In record time, two large randomised trials of stent vs balloon angioplasty were organised and carried out. STRESS in the United States and BENESTENT in Europe and South America. The results of both trials showed, for the first time, lower restenosis rates for stents than for balloon angioplasty11,12. A second presentation to the FDA, this time by Patrick Serruys, met with approval, and the coronary stent became available in the US “to reduce restenosis of balloon angioplasty” in August of 1994.
Even though a large clinical experience was available in peripheral and coronary applications, in 1994 there were many uncertainties about the long-term fate of implanted stents. By then, long-term dog coronary implants were available indicating that a metal stent could remain intact many years after implantation. However in my mind, things could still go wrong in the very long term. As a resident, I had the opportunity to come across a patient who may have been the last survivor of a Hufnagel valve, the very first aortic valve developed shortly after WWII. This patient, as most other recipients of the pioneering device, had developed a large thoracic aorta pseudoaneurysm. We reported the finding13, and it became a fear that haunted me, that untoward effects could take place many years after an implant. The results of the balloon-expandable stent, as far as I could see in 1995, were very exciting. However, early clinical experience showed a small but consistent percentage of patients suffering from stent thrombosis during the first three weeks after implantation, despite the use of coumadin and aspirin. Fortunately, the use of intravascular ultrasound and high pressure balloon inflation improved the results of stenting14. Later, clinical investigation showed that coumadin could be dispensed with in favour of thienopyridines15.
During 1995, stent usage started to escalate. The concern that stenting would be reserved to operators with special skills and expertise was dispelled, as eventually every interventionalist received trained in the techniques of stenting. The adoption of rapid exchange catheter technology into stent delivery systems, made stents easier to handle.
Litigation years
The rate of stent use in percutaneous coronary revascularisation went from 10 to 70% in a few years. Johnson and Johnson, after being practically alone in the market place for two years, suddenly saw the appearance of several competitors coming up with new balloon-expandable stents. In late 1996, the market share of JJIS plunged from 95% to 5% in weeks. This wrecked havoc on the small subsidiary that had pioneered the new technique, at a time when their production was recently scaled up to the maximum to meet the growing demand. The recently expanded work force at JJIS had to be dismantled and large layoffs took place.
Some were critical of the way JJIS conducted business, particularly in regard to the pricing policies16. On the other hand, it had taken almost ten years for JJIS to get any return for their investment and, as the pioneer, they had to create the infrastructure to educate the physicians in the new technique. Also, they had to lead the way in getting reimbursement for the new procedure from the government. The competition took advantage of this JJIS burden and penetrated the market stealthily with new products having the advantages of the brand new technical methods available: computer assisted design and laser manufacturing. Without the need to repeat randomised comparison studies with balloon angioplasty, the new stents were approved if they demonstrated “equivalency” to the PS stent.
Johnson and Johnson felt that the new stent designs were based on intellectual property they owned and prepared for a comprehensive legal claim over their infringed rights and loses. As the author of the patents on balloon-expandable stents I got drafted into prolonged court disputes over patent rights, first in Europe and later in the US.
I spent hundred of hours in tedious depositions and many days in courtrooms. Since the litigating companies were incorporated in Delaware, the Dupont hotel in Wilmington became a very familiar place for me. This hotel was adopted as headquarters by the Patterson Belknap law firm. The main litigator for Johnson and Johnson, Craig Diskant and the medical expert doctor Nigel Buller from the UK, made the dreadfully boring days in court as interesting as it could possibly be. At the end, I was even enjoying some of the fascinating aspects of the US legal system and making my own comparison with the legal proceedings in Canadian, Dutch and British courts.
Yesterday, today and tomorrow
The new millennium brought with it the DES or drug-eluting stent. This new approach brought a practical way to decrease stent restenosis to acceptable levels, at a time when the mechanisms of restenosis are not completely understood. However, this stent restenosis – a vexing problem for both patient and interventionalist – seems to have been solved by DES.
Adverse effects with DES, such as late thrombosis and hypersensitivity reactions are of concern but fortunately, they remain rare. However, recently the press seems to be keenly focused on these negative aspects of the DES while everybody takes the advantages of DES for granted. A fact of life, I guess.
Interestingly, the results with bare metal stents are getting better, making cost considerations all the more important, as more patients and more lesions per patient are being treated with stents.
From the perspective of the early eighties the wide adoption of stents, and percutaneous revascularisation in general, was impossible to predict. The balloon-expandable patent was hailed as one of the “Ten patents that changed the world”17. Soon after this article was published, I was invited to donate my early stent prototypes and jigs to the medical collection of the Smithsonian Institution, and the balloon-expandable stent got a display at the Texas History Museum in Austin, Texas. I also was inducted in 2006 into the National Inventors Hall of Fame in Akron, Ohio. Just thinking who are in the list of inductees gives me goose bumps.
Although I am profoundly honoured by all these recognitions, the early ones are particularly meaningful to me because of the great support and encouragement they gave me at times when I really needed it. The earliest recognitions I received were the Lifetime Achievement Award at the Texas Heart Institute in Houston, Texas in 1996 and the Andreas Gruntzig Ethica Award to the Best Inventor at the Thoraxcenter, Rotterdam 1997.
The stent project surprised me with unexpected success many times. However, the largest surprise for me came three years ago. While I was working out just before going to work at 6:00 am, I felt a poke in the chest that went away with rest and returned with exercise. A stress-thallium scan that morning showed an anteroseptal defect that correlated with a known calcification in my proximal LAD. The next day, Steven Bailey placed a stent which was dilated to 4 mm to cover a lesion 13 mm long. The experience of being a stent patient was nothing short of fascinating, and made me think how lucky I am to be living in this era. My own father died of a heart attack at my present age, and my father-in-law died during a gruesome bypass surgery thirty years ago. After my procedure I went back to work in two days and was running in a week!
These days I spend time in my newly found passion of wine making. In the peaceful Napa Valley I have time to reflect on how good the balloon-expandable stent has been to me and to millions of other people.

