The dynamic interaction of the surface of cardiovascular stents with the vessel wall and other intravascular biological components plays a key role in the final outcome seen after device implantation (mainly restenosis and thrombosis). While the use of currently available bare metal stents has a reported restenosis rate of 20-30%, the use of the more recently developed drug-eluting stent has reduced this rate to less than 10%. The current therapeutic strategy employed with these drug-eluting stents is designed to limit the growth of neointima by either cytostatic or cytotoxic effects on proliferating arterial wall smooth muscle cells1,2. Unfortunately, both the eluted drug agents as well as the polymer base from which they elute disrupt the natural healing response thereby delaying endothelialisation and leaving the stented area prone to late thrombosis3. An emerging concept is to limit stent restenosis and thrombosis by modifying the stent surface, thus encouraging the rapid restoration of a healthy functioning endothelium. An intact endothelium residing within an arterial flow environment is antithrombotic, inhibits leukocyte recruitment, and secretes agents including nitric oxide that control the underlying arterial wall architecture by limiting arterial wall SMC migration and proliferation4.
Endothelial healing at sites of stent or vascular graft implantation occurs principally by cell migration from adjacent areas of intact endothelium5. Recent reports suggest that blood derived circulating endothelial progenitor cells and their interaction with platelets6 may also contribute to re-endothelialisation at these sites. Surface modification may be achieved by a) optimising the electro-chemical properties, b) structurally modifying the topography or c) adhering biomimetic molecules on the stent surface. Several surface modification strategies have been designed to enhance endothelial cell attachment as well as migration onto and across the stent surface. Surface texture modifications that have been most advantageous in encouraging endothelialisation are those that create topological features that are subcellular in dimension. Recent creation of a nano-structured titanium surface, achieved by compressing titanium nano-particles into a titanium surface to increase surface roughness, was demonstrated to enhance endothelial adherence by nearly 4-fold7. In an attempt to direct endothelial migration, groove patterned surfaces have been created on stainless steel and titanium surfaces. Using a novel etching technology to create groove widths ranging from 750 nm to 100 µm, Lu et al observed a 2-fold increase in endothelial adherence at groove sizes less than 2 µm8. Palmaz et al demonstrated a more than a 2-fold increase in endothelial cell migration rate on to and across stainless steel stent material patterned with microgroove widths of 22 µm or less9. Though these laboratory results are encouraging, these surface modifications have yet to be evaluated for their ability to encourage endothelialisation in preclinical animal studies. Alterations in surface morphology can also be exploited to indirectly influence endothelialisation or limit restenosis by creating features that function as drug repositories, thereby eliminating the need for a polymer coating. Such a microporous surface structure has been created using a carbon-carbon deposition on a cobalt-chromium stent base10. Bhargava et al reported that these stents, in this case loaded with tacrolimus, both limited restenosis and exhibited an intact endothelium compared to Cypher stents when implanted in porcine coronary arteries. This approach would also seem attractive to deliver agents, eg., FGF or VEGF, that would potentially increase endothelial migration to stented sites.
Endothelial cells not only respond to physical surface topography but also surface chemical cues. Endothelialisation rates vary greatly when different metal surfaces are compared. For example, endothelial migration on to stainless steel is significantly greater than that observed on gold surfaces11. Direct chemical modification of stent surfaces has yielded mixed results on endothelial cell adherence and/or function. Specifically, we have found that while similar endothelial coverage may be observed on different treated nitinol surfaces, the endothelial functionality may differ greatly (Figure 1). Using a low temperature plasma deposition technique to modify a nitinol stent surface with or without a subsequent treatment of the surface with a TiO2 sol, Wang et al report a surface with increased affinity for endothelial cell adherence12,13. In contrast, increasing nitrides on a 316L stainless steel stent surface using a low temperature gas plasma deposition process failed to demonstrate any increased cell adherence compared to untreated control stent surfaces14. However, osteoblast rather than endothelial cell adherence was tested.
Stents remain attractive devices not only to reopen occluded arterial lesion sites but also to act as drug delivery vehicles. There is increased interest and development of using surface modifications to directly link therapeutic agents to the stent surface without an intermediary polymer. Sergeant et al report a method that produces an aminated nitinol surface suitable for covalent attachment of self-assembled peptide amphiphile nanofibres with bioactive functions15. Using this method to attach endothelial adhesion peptides, a surface with increased affinity for endothelial cells was created. Stainless steel coronary stent surfaces have been modified with the attachment of the linker molecule, Hexamethylene diisocyanate (HMDI), to allow attachment of heparin or other bioactive peptides16. In an effort to rapidly endothelialise implanted stents by capturing circulating endothelial progenitor cells (EPCs), stainless steel stents have been coated with an antibody that binds the CD34 molecule specifically expressed on EPCs17. The first-in-man initial clinical trials with this novel stent have been completed. Some recent studies indicate that recruitment of EPCs to re-endothelialise vascular interventional sites may not result in establishment of the same functioning endothelium as that obtained from migration of adjacent mature endothelial cells. Enhanced intimal hyperplasia, in fact, has been reported with the seeding of EPCs18-20.
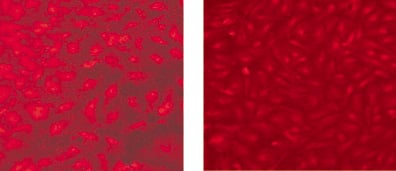
Figure 1. Human aortic endothelial cells immunostained for expression of the pro-inflammatory marker, vascular cell adhesion molecule (VCAM-1), that have migrated on to a rough textured, electropolished nitinol surface (left panel)) compared to cells that have migrated on to a modified surface chemistry treated nitinol surface (right panel). Note that though both surfaces exhibit cell coverage, the cells on the modified surface chemistry exhibit optimal confluent cell morphology with no VCAM-1 expression.
In conclusion, the biological optimisation of the stent surface represents vast opportunities as a means of encouraging and accelerating the natural healing response to the requisite injury associated with balloon-expandable stent implantation. Rapid restoration of an intact, functional endothelium at these interventional sites has the potential to greatly reduce late adverse cardiovascular events associated with current DES technology and limit the long-term use of antiplatelet agents. Finally, stent surface modification potentially enables the delivery of selective agents tailored to specific clinical needs, such as delivery of agents that target the inflammatory component, as well as agents that promote endothelial healing in patients suffering from acute coronary syndrome, might be particularly advantageous.

