Abstract
Aims: Small Intestine Submucosa (SIS) has been investigated in experimental cardiac valve replacements for its unique ability to remodel. One of its main problems however is uncontrolled remodelling leading to increased leaflet thickness, which finally results in valvular dysfunction. This study aims at investigating a percutaneously placed self-centring SIS valve in the porcine pulmonary artery in order to study its influence on reduction of leaflet thickening.
Methods and results: Five male Yucatan pigs were implanted a self-centring SIS valve construction into the pulmonary artery. The pigs were followed for six months post implant with angiography and histologic evaluation. There were no misplacements during deployment. Angiography showed all except one valves functional. Histology demonstrated intensive remodelling, imitating the tissue architecture of the native valve. Inflammation and leaflet thickening was minimal, however 77% of the valve leaflet lengths adhered to the arterial wall. Arterial wall thinning was present in the area of the valvular graft placement.
Conclusion: While the SIS tissue has remarkable properties that have the potential to significantly prolong graft longevity, the current valve designs are not suited to fully utilize this potential and need further improvement to optimize rheology.
Introduction
Valvular heart disease accounts for approximately one third of all non-ischaemic heart disease1, and surgical replacement remains the cornerstone of its therapy2. The past 20 years have brought remarkable improvement in valvular heart disease, including advances in prosthetic valve development and minimal invasive surgical techniques3. There are two principal types of valve prostheses, mechanical and biological. Mechanical valves are associated with substantial risks of thromboembolism and haemolysis; whereas biological valves tend to become dysfunctional in a relatively shorter time due to tissue deterioration4-7. Their indication is restricted to patients with either life expectancies shorter than the time interval to repetitive surgery, when the risks of anticoagulation predominate the benefits of durability, or whenever anticoagulation is not feasible8,9.
Currently, a significant amount of research is dedicated to improve biological valves endurance by reducing inflammation, calcification, and thromboembolism. Therefore, small intestine submucosa (SIS) has been introduced into valve replacement as a potential biological valve. SIS has been shown to induce variable degrees of tissue-specific remodelling in the organ or tissue into which it is placed10-19. SIS bio-engineered tissue substitutes have been applied in urologic and orthopaedic interventions before, which levelled their way into cardiology and vascular surgery due to their encouraging data in the non-cardiologic fields12,14. With regard to valvular replacement however, the data remain contrary. Up until now, SIS material has been applied in porcine, canine, and sheep models10,11,13,17,19-22. Some of the data indicate encouraging results in regard to prolonged graft endurance due to tissue remodelling, growth adaptation, and the option of percutaneous delivery. Recently, we have reported a valve based on porcine small intestine submucosa (SIS) used as a xenograft material23. We have shown that a SIS valve does induce remodelling, however, this process was uncontrollable and long term results at one year showed increased valve thickness23. We hypothesized that discordance of the valve position within the pulmonary artery leading to increased resistance to the blood stream was a key mechanism of inducing ongoing thickening of the valve leaflets, thus resulting in loss of function. Therefore, we further developed the design of the valve to resolve the problems arising from malalignment and investigated a percutaneously placed self-centring SIS valve.
Methods
This investigation was approved by the University of Illinois at Chicago’s Animal Care Committee, and was in compliance with the 1996 Guide for the Care and Use of Laboratory Animals and with the Animal Welfare Act.
Animal model
Five mature male Yucatan miniature swine (44-62 kg, above 2 years of age) were implanted with one prototype prosthetic venous valve in either the left or right pulmonary artery for six months. The animals were on standard laboratory swine diets with no lipid or cholesterol supplementation. Water was provided ad libidum, and the animals were maintained in indoor, raised-floor pens. All animals were pre-medicated with aspirin (325 mg daily) beginning three days prior to surgery. On the day of surgery, the animals were pre-medicated with a mixture of ketamine and acepromazine (15 and 0.15 mg/kg intramuscularly, respectively) to induce anaesthesia. The pigs were then intubated and gas anaesthesia was maintained at stage III with 1-3% Isoflurane (Iso-thesia, Burns Veterinary Supply, Rockville Center, NY) in oxygen. Butorphanol (0.01 mg/kg intramuscularly) was administered for analgesia.
Self-centring SIS valve
The self-centring SIS pulmonary valve prostheses were constructed of square stents with four nitinol barbs (Cook Inc., Bloomington, IN), interlinked with steel wire forming a rhomb-like outer ring carrying the main frame of the valve. This construction allows the SIS valve bearing wire to gear out to the main axis of the blood stream in order to reach a minimum of resistance. The inner square stent is simply constructed with four corners bent into spring-like coils to reduce stress and metal fatigue17. The wire ends are extended 1 mm over the stent frame forming barbs on opposing stent corners to provide anchors for the stent during placement. Two more anchoring barbs are added by attaching a wire to the contra-lateral side of the stent frame. Two triangular pieces of SIS were sutured with 7.0 Prolene monofilament to the inner steel construction (Ethicon, Somerville, NJ). The layer of SIS sutured to the metal frame had a flap about 2 mm wide extending beyond the frame11,20. All valves were 12 mm in diameter. After construction, the valves were lyophilised and gas sterilized. The lyophilised SIS sheet was 100-120 µm thick. The valves were then folded and front loaded into an 8 Fr Teflon sheath 80 cm long (Cook Inc, Bloomington, IN) and delivered coaxially (Figure 1).
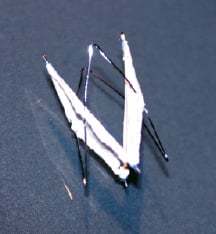
Figure 1. Self-centring Small Intestinal Submucosa valve.
Percutaneous valve implantation
The surgical sites were scrubbed and the jugular vein was exposed via cutdown. A 9 Fr introducer sheath was placed in the jugular vein to gain venous access and the animal was administered heparin in a bolus of 200-300 IU/kg. A 5 Fr, selective, pigtail angiographic catheter was advanced over an Amplatz wire (AGA Medical Corp, Golden Valley, MD) under fluoroscopic guidance into the pulmonary artery. A non-ionic contrast agent, ioversol (Optiray; Mallinckrodt, St. Louis, MO), was injected by hand to obtain adequate filling for vessel sizing. A graduated wire guide was inserted through the pigtail catheter and advanced sufficiently to allow imaging of the graduated wire guide within the catheter. The diameter of the pulmonary artery was measured using the distance between radiopaque bands on the graduated wire guide as a calibration reference. The self-centring SIS valves were placed in a segment of the pulmonary artery measuring 11.5 mm-13.5 mm. Each prosthetic valve was delivered to the selected implantation site under fluoroscopic guidance, and deployed. Immediately following implantation, a pigtail catheter was re-introduced over the guide wire to the pulmonary artery and angiograms were taken to assess the patency of the valve. Following post-deployment angiography, the femoral vein was ligated, the incision closed and the animal recovered in a raised floor pen. The animals continued to receive aspirin (325 mg, daily) for the duration of the study.
Follow-up
Upon six months after the valve placement, the animals were prepared and the contralateral jugular vein accessed as in the implant procedure. Following placement of the venous introducer sheath, the pigs were administered a bolus of 200-300 IU/kg heparin. Angiograms of the pulmonary artery were taken, as previously described, to evaluate valve position and patency. Following angiography, the animals were euthanized with an intravenous administration of saturated potassium chloride. After euthanasia, a thoracotomy was performed to expose the pulmonary artery. The section of pulmonary artery, containing the valve, was excised with the inclusion of approximately 10 mm of reference vessel beyond both the proximal and distal ends of the valve.
Histology
The excised pulmonary artery segments were placed in 10% neutral buffered formalin immediately after they were obtained and were allowed to fix for 24-48 h. After fixation, the samples were dehydrated and embedded in methylmethacrylate plastic. After polymerization, the samples were cut on a plane right-angled to the tissue surface. Longitudinal sections of 4 µm were cut with a rotary saw microtome and transferred onto a microscopic slide. For histological examination, sections of tissue were deparaffinized in four cycles of Xylol for 10 min. each. The samples were rehydrated in aqua dest for 10 min, and subsequently stained with the Hematoxylin-Eosin method24. A board-certified veterinary pathologist, experienced in the evaluation of chronically implanted vascular stents, performed qualitative histopathologic evaluation of the stented vessels. Slides were evaluated for remodelling of the valve material and to assess potential adverse effects with particular attention to interface between the implant and the vessel wall, as well as signs of graft rejection.
Results
The self-centring SIS valve was percutaneously implanted in all five pigs (Figure 2a).
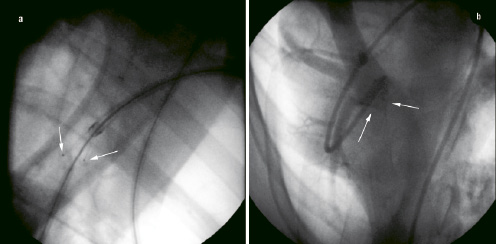
Figure 2. Angiography (a): Placement of a self-centring valve in the pulmonary artery. The tips of the valve leaflets are marked radio-opaque (white arrow). (b): Functional assessment at six months follow up, an angiography catheter is advanced over the superior vena cava beyond the implanted valve, and contrast is released. The valve is occlusive, no contrast is leaking back to the main pulmonary artery.
There were no major clinical complications from any of the procedures and nor were any observed during the follow-up period (i.e. valve thrombosis, infective endocarditis).
Function
Upon six months follow up, valvular function was assessed prior to removal via an angiocardiography. All valves were functional (Figure 2b), except one of the animal that revealed evidence of moderate valvular insufficiency on angiography.
Valve hinge region
In all valves, inflammatory changes at the valve base were minimal and consisted of scattered lymphocytes within connective tissue around sutures and struts. SIS material was not visible at the valve base. Mature fibrovascular tissue filled in the space created by the stretching of the arterial wall behind the struts at the valve base. Additionally, at each base strut, the arterial wall was moderately compressed and bulged outwardly. The wall was thinned to approximately 100 µm, and sometimes to <100 µm, at the base strut. In these areas, the wall was composed of dense fibrous tissue with some smooth muscle. One base strut had a tiny clump of amorphous SIS collagen that was surrounded by granulomatous inflammation (Figure 3a).
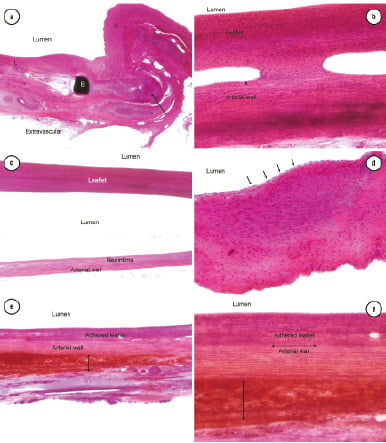
Figure 3. Histologic evaluation of the self-centring SIS valve at six months post implantation, haematoxylin eosin staining (a): 40x magnification, granulomatous inflammation around amorphous material at base (arrow). Base strut (b). Leaflet (l) overlies the arterial wall. (b): 100x magnification; firm focal adhesion between the arterial wall and leaflet. Abundant spindle-shaped host cells infiltrate the leaflet. (c): 40x magnification; densely cellular, non-adherent leaflet and adjacent arterial wall with thin layer of neointima. (d): 200x magnification; spindle-shaped cells of at leaflet tip resembling smooth muscle cells. Arrows outline complete endothelialisation of leaflet. (e): 40x magnification; leaflet firmly adhered to arterial wall. Haemorrhage (arrow) outside of arterial wall is acute and interpreted as an artefact of post-mortem collection. (f): 100x magnification: details of the adhesion region in (e) (dotted arrow) between the arterial wall and leaflet. Region of post-mortem haemorrhage around artery (solid arrow).
Leaflet adhesions
Overall, 13%-100% (average 77%) of leaflet lengths, which ranged from 15-23 mm (average 19.7 mm) adhered to the arterial walls. Sometimes, there were multiple adhesion sites between the leaflet and underlying arterial wall (Figure 3b). Leaflets with the adjacent incorporated wall ranged from 1.0-2.5 mm thick. Leaflets were relatively uniform in thickness. At mid leaflet, free (non-adherent) leaflets ranged from 0.1-0.35 mm thick (Figure 3c).
Remodelling
In all valves, mature endothelium completely lined the peripheral and non-adherent central surfaces of leaflets (Figure 3d). Leaflets were densely cellular and composed of spindle-shaped cells resembling smooth muscle that were generally longitudinally oriented along the long axis of the leaflet (Figure 3c, d). In most leaflets, a small population (usually <50% of cells) of spindle-shaped cells intermixed with the longitudinally oriented smooth muscle cells. These cells were slightly more haphazardly oriented and had less cytoplasm suggesting a fibroblastic phenotype. In this layer, tiny collagen fibres were rarely present. Most leaflets contained occasional capillaries and fewer larger vascular channels. Inflammatory changes were minimal to absent.
Arterial wall thinning
In general, arterial walls were minimally compressed and minimally thinned within the length of the valve compared to the artery wall outside of the valve. The thinned smooth muscle was otherwise unremarkable. At most sites of adhesion between the valve leaflet and arterial wall, valve leaflets blended seamlessly with the arterial wall forming a firm adhesion (Figure 3e, f). Arterial walls beneath unattached leaflets had a thin layer of mature neointima lining the lumen.
Discussion
Biologic valve replacement has been applied for more than thirty years, however, improvement of graft endurance is still far from desired durability and long-term performance with only marginal improvement25,26. Valvular degradation occurs by inflammation, mechanical wear out, calcification, and enzymatic digestion with subsequent loss of histologic integrity27-29. Recent works on SIS valves suggest that it provides substantial improvement in many aspects associated with early tissue remodelling as well as lack of significant immunologic reaction, suggesting prolonged graft survival30,31. The ability of SIS to remodel and thereby to emulate histologic properties of a native heart valve, including the presence of endothelial cells, fibroblasts, and smooth muscle cells, were previously documented23. However, despite similarities in histological appearance, the remodelled SIS valve does not replicate the functional anatomy of a native heart valve32.
Despite the encouraging results demonstrating SIS somewhat resembles a native valve, by the third month after implantation the process of tissue remodelling has been uncontrollable, leading to continuous valve leaflet thickening and eventual loss of function. The SIS is a very reactive tissue that responds to many external stimuli10-19. The self-centring valve design attempts to address the problem of malalignment of the orifice of the valve with the vessel, and this can be a major source of rheological abnormalities compared to a native valve. This abnormal blood flow may be a very important stimulus to explain the uncontrolled remodelling and therefore by evading this potential stressor leading to continuous leaflet thickening. However, despite some of the histology suggesting less valve leaflet thickening compared to the previous SIS valve construction23, the self-centring valve design still had excessive thickening and in addition it revealed to have a number of different problems, such as attachments and subsequent fusions of its leaflets with the arterial wall. These valve leaflet attachments do lead to loss of function33,34. Another problem observed with the self-centring design is the external pressure exerted by the frame on the arterial wall, induced wall thinning at the anchoring points. The construction of the device requires a balance between adjacency to the arterial wall and pressure induced damage of the arterial wall. Recently, Pavcnic et al. stressed the importance of proper spatial orientation of percutaneously placed valve replacements at the time of deployment, which should be as close as possible to that of the original native valves, as an important factor for valve function35.
Until now, surgical procedures still make up for the vast majority of valve replacements36,37, despite recent advances in percutaneous valve replacement. However if this new technology is to be offered to patients with valvular heart disease, these prosthesis will need to be, not only safer than the current surgical gold standard, but that they will be able to last at least as long as the current biological prosthesis being implanted today.
In conclusion, the current data on percutaneously placed SIS valves remains ambiguous. They support the ability of SIS valves to remodel, which could potentially solve numerous problems limiting graft endurance of many biological valve prostheses. However, at this present time, the benefit of remodelling cannot be translated into a clinical advantage as long as problems such as uncontrollable remodelling cannot be resolved. We hypothesize that the problems encountered are not to be attributed to the properties of the SIS tissue itself, since it only reacts to ongoing stimulus, but rather to the valve-support system and valve design. The self-centring valve design might improve the rheological characteristics of the SIS valve and thereby reduce leaflet thickening. However, the present valve design needs further improvement to avoid the problems of leaflet attachments and arterial wall thinning as well as better control of the remodelling process. Therefore, ongoing investigations need to focus primarily on the problems associated with the valve frame design to utilize the potential benefits of SIS based valves in the future.
Acknowledgements
This study was supported in part by a grant from Cook, Inc.

