Abstract
Objectives: The STEALTH (STent Eluting A9 BioLimus Trial in Humans) trial was the first-in-man study to assess the safety and efficacy of the bioabsorbable-polymer-coated Biolimus A9™-eluting BioMATRIX™-Stent, as compared to a bare metal stent control (S-Stent™).
Methods: One hundred twenty patients were enrolled in a prospective, 2:1 randomised, double-blind, multi-center clinical study. Patients with single de novo coronary lesions received either a Biolimus-eluting stent (n=80 patients with 82 lesions) or bare metal control stent (n=40 patients with 40 lesions). The primary study endpoint was in-lesion late lumen loss at 6 month follow-up.
Results: The six month in-lesion late loss was significantly reduced in the Biolimus-eluting stent group (0.14 ± 0.45mm) as compared to the control (0.40 ± 0.41mm) (p=0.004). The in-stent late loss was 0.26 ± 0.43mm in the Biolimus-eluting stent group vs. 0.74 ± 0.45mm in the control (p<0.001). The secondary study endpoint of event-free survival at 6-months was similar in both groups (96.3% Biolimus group vs. 97.5% control; p=0.72). There was no significant difference in target lesion revascularizations due to the low binary restenosis rates in both groups (3.9% in Biolimus group vs. 7.7% in control; p=ns). Conclusions: In this first-in-man feasibility trial, the bioabsorbable-polymer-coated Biolimus A9™-eluting stent demonstrated superior efficacy in reducing 6-month in-stent and in-lesion late loss and percent diameter stenosis, with clinical safety similar to bare metal control stents.
ABBREVIATIONS
STEALTH: STent Eluting A9 BioLimus Trial in Humans
PLA: polylactic acid
MACE: major adverse cardiac event
CABG: coronary artery bypass graft surgery
MLD: minimal lumen diameter
LAD: left anterior descending artery
LCX: left circumflex artery
RCA: right coronary artery
NS: non significant
MI: myocardial infarction
PCI: percutaneous coronary intervention
TLR: target lesion revascularization
TVR: target vessel revascularization
Introduction
The advent of drug-eluting stents has revolutionised the treatment of coronary artery disease. Randomised controlled trials of sirolimus-eluting1-6 or paclitaxel-eluting stents7,8 demonstrate that they significantly reduce neointimal hyperplasia formation when compared to bare metal stents, as evidenced by reduced angiographic late loss and binary restenosis rate. This results in improved clinical outcomes with lower target vessel or lesion revascularisation rates.
Sirolimus derivatives are being evaluated for use on drug-eluting stents for potentially superior elution characteristics, tissue distribution, or restenosis inhibition properties. Biolimus A9™ is a new sirolimus derivative that binds to the cytosolic immunophilin FK506-binding protein 12 (FKBP12) and inhibits growth factor-driven cell proliferation, including T-cells and vascular smooth muscle cells9. It has the potential in coronary stents to prevent restenosis by interrupting smooth muscle cell migration and proliferation. In addition, an enhanced lipophilicity enables an increased uptake in local target tissues and reduced presence in the systemic circulation.
The aim of this first-in-man STEALTH (STent Eluting A9 BioLimus Trial in Humans) trial was to evaluate the safety and efficacy of a Biolimus A9(tm)-eluting stent with a bioabsorbable coating as compared to a similar bare metal control stent.
Methods
Study design
This study was a multi-center, prospective, double-blind, randomised controlled trial. The study design randomly assigned 120 patients undergoing coronary angioplasty in a 2:1 proportion to the BioMATRIX™-Stent group and the control S-Stent group, respectively. Patient enrollment in 3 centers (Heart Center Siegburg, Germany (n=50), the Institute Dante Pazzanese of Cardiology, São Paulo, Brazil (n=48) and Brüderkrankenhaus Trier, Germany (n=22)) began in September 2003 and ended March 2004.
Study endpoints
The primary endpoint was the quantitative angiographic in-lesion late loss, defined as the minimum lumen diameter (MLD) in a stent-treated segment minus the MLD of the same region at follow-up. The main secondary endpoint was event-free survival, defined as the absence of combined major adverse cardiac events (MACE) such as death, myocardial infarction (MI), coronary artery bypass graft (CABG) surgery, or repeat percutaneous intervention procedure to the target lesion during the follow-up period. Other secondary endpoints included the occurrence of MACE and reduction in binary restenosis at 6-month follow-up. Device success was defined as successful stent delivery, and clinical success was defined as device success without procedural events.
Stent characteristics
The BioMATRIX™-Stent (Biosensors International, Singapore) comprises a stainless steel, corrugated ring, quadrature-link design, balloon expandable S-Stent, and a polylactic acid (PLA) polymer/Biolimus A9™ coating. The coating is ~15 µm thick, comprising a 1:1 combination by weight of Biolimus A9™ and PLA, delivering a standard dose of 15.6 µg of drug per mm of stent length. The coating is asymmetric, with the drug and PLA biased to the abluminal (outside) surface of the stent to allow greater localised drug delivery and to reduce systemic release as well as drug anti-proliferative effects on cells lining the inside of the stent lumen. PLA was selected for stent use because it is bioabsorbable, breaking down initially into low molecular weight polylactic acid, and then subsequently into carbon dioxide and water concurrent with drug release. The PLA coating has a high drug-carrying capacity, which minimizes polymer coating weight and potential for foreign body response and inflammation from the polymer. Stents were available in varying lengths (8 mm, 14 mm, 18 mm and 28 mm) and stent diameters (2.5 mm, 3.0 mm, 3.5 mm and 4.0 mm). The average strut thickness for the stent was 0.0046 inches. The control stent arm of the study used a bare metal S-Stent which forms the metal backbone of the BioMATRIX™ drug eluting stent.
Patient inclusion / exclusion criteria
Patient inclusion criteria included age >18, native coronary artery with a reference diameter ≥ 2.75 mm and ≤ 4.0 mm, a lesion stenosis ≥ 50% and ≤ 99%, and lesion length ≤ 24 mm. Additionally, patients were required to have symptoms of angina or ischemia, and to be acceptable candidates for CABG. All patients were required to provide informed consent prior to study enrollment and to agree to the follow-up investigations. Major exclusion criteria included the presence of a side branch with a diameter >2 mm, prior stenting of the target lesion, angiographic evidence of thrombus or poor distal flow, significant medical comorbidities, left ventricular ejection fraction <30%, stenosis >50% of the left main coronary artery, indication for insertion of more than 2 stents, contraindication or known hypersensitivity to anticoagulants or sirolimus or its derivatives, stroke or transient ischemic neurologic attack (TIA) within the prior 7 days, or an acute MI within the previous 7 days.
Procedural characteristics
A loading dose of heparin (75 units/kg) was administered intravenously to each patient to maintain the activated clotting time (ACT) level at >250 seconds during the procedure. ACT was maintained between 225 and 300 seconds for patients administered a glycoprotein IIb/IIIa antagonist. Patients were administered a loading dose of clopidogrel (300 mg) or ticlopidine (500 mg), as well as aspirin (300 mg) for patients not already on aspirin. Each target lesion was predilated by conventional balloon angioplasty using a balloon at least 4 mm shorter than the stent length. Direct stenting was not allowed. Stenting was performed using a deployment pressure to achieve a final balloon diameter of 105% to 110% of the reference vessel diameter (RVD), and a residual diameter stenosis of <20%. Post-dilations were performed at the discretion of the operator using short balloons confined within the stent boundaries.
Standard drug therapy was followed post procedure. Patients received at least 125 mg aspirin twice daily, together with clopidogrel 75 mg/day for three months or 250 mg ticlopidine twice daily for two weeks only. Concomitant medication was prescribed at the physician’s discretion. Clinical follow-up evaluations were conducted at 48 hours, 1 month, 3 months, and 6 months post stent implantation. The 6-month follow-up also included an intravascular ultrasound (IVUS) and a control coronary angiography.
Quantitative angiographic measurements
After intracoronary nitrate administration, 2 or more orthogonal views to show non-overlapping and non-foreshortened views of the target lesion were taken. Quantitative angiographic endpoints included MLD, percent diameter stenosis (%DS), late lumen loss, and binary restenosis (defined as >50% diameter stenosis). Acute gain was defined as the change in MLD from baseline to post-intervention. In-lesion measurements included the stented segment as well as the margins 5 mm proximal and distal to the stent. The angiographic data were independently analyzed by the Cardiovascular Research Foundation Angiographic Core Lab (New York, NY, USA).
Statistical analysis
Data are presented as mean ± SD or frequencies. Statistical analyses were conducted by the Harvard Clinical Research Institute (Boston, MA, USA). Data were analyzed and reported on an intent-to-treat basis through 6-month follow-up. Categorical data were compared with Fisher’s exact test and continuous variables with t tests. A P value of <0.05 was considered significant.
Results
One hundred twenty patients were recruited in this trial. Baseline clinical characteristics were similar between the patients treated with the BioMATRIX™-Stent (n=80 with 82 lesions) and the S-stent (n=40 with 40 lesions), although there were fewer male patients in the Biolimus stent group (Table 1). Diabetic patients accounted for 26.6% of the patients in the drug-eluting stent group. Peak Biolimus A9™ peripheral blood levels of <3 ng/ml (a concentration 10-fold less than the drug’s observed level of no systemic clinical effect) were detected immediately after stent implantation, and within three days thereafter rapidly dropped to below the detection limit (0.5 ng/ml).
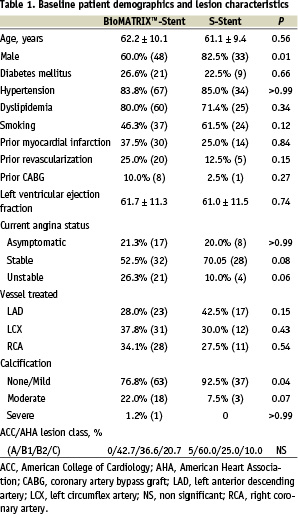
Angiographic results
There was no significant difference in the baseline angiographic lesion characteristics between the two groups (Table 2). Six month clinical and angiographic follow-up was performed for 95% (114/120) of patients. At 6-months post procedure, a significant 65% reduction in in-stent late loss (0.26 mm vs. 0.74 mm; p<0.001) as well as in-lesion late loss (0.14 mm vs. 0.40 mm; p=0.004) was observed in the Biolimus stent group as compared to the control (Figure 1). Binary restenosis rates did not differ significantly between the Biolimus stent and the control groups, with low rates in both groups (3.9% vs. 7.7% respectively for both in-stent and in-lesion rates; p=0.40).
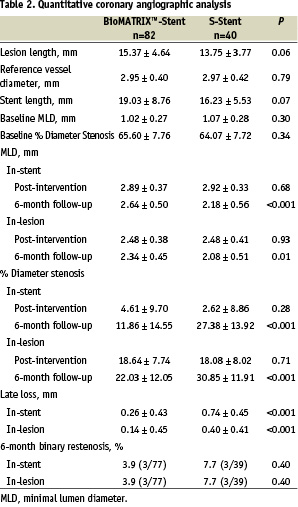
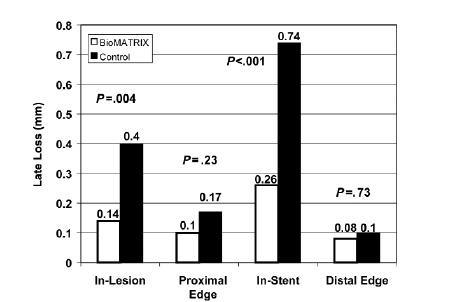
Fig. 1: Angiographically determined late loss at 6-months in patients treated with the BioMATRIX™-Stent or the S-Stent. Significantly less in-lesion (P=.004) and in-stent (P<.001) late loss was observed in patients treated with the BioMATRIX™-Stent.
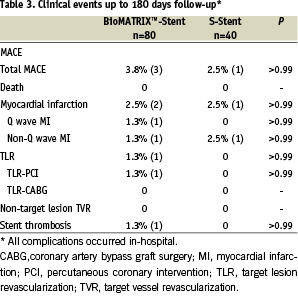
Clinical results
Device success in the Biolimus-eluting stent and control group was 98.8% (79/80) and 100% (40/40), respectively (p>0.99). Clinical success was 96.3% (77/80) in the Biolimus-stent group and 97.5% (39/40) in the control (P>0.99). The event (MACE)-free survival was similar for both groups at 6 months (96.3% Biolimus stent vs. 97.5% control; P=0.72). TLR-free survival was 98.8% for the Biolimus-eluting stent group and 100% for the control bare metal stent (p=0.48). Total MACE rates were low and similar for patients in both groups (3.8 % in Biolimus stent group and 2.5% in control group) (Table 3). There were no deaths, and no patients required an emergent CABG surgery. There was one acute stent thrombosis in the Biolimus-eluting stent group at the day of implantation, successfully treated by repeated balloon angioplasty. All clinical complications occurred in-hospital, and no clinical complications occurred during the follow-up period up to 6 months.
Discussion
Biolimus A9™, a new sirolimus derivative, is modified at position 40 of the rapamycin ring to enhance its lipophilicity and elution rate relative to the parent drug, resulting in an increased uptake in local target tissues and therefore a reduced presence in the systemic circulation. Implantation of a Biolimus-eluting stent in a swine low-overstretch injury model showed a >40% reduction in vessel restenosis compared to a bare S-Stent control9. The results of this first-in-man trial demonstrate that the use of the bioabsorbable-polymer-coated Biolimus A9™-eluting stent in de novo coronary lesions is safe and effectively suppresses neointimal growth, as demonstrated by a significantly better in-stent and in-lesion late loss and %DS relative to a similar bare metal stent at 6-month follow-up.
Various sirolimus derivatives recently have been evaluated for use on drug-eluting stents in order to find an agent with improved elution and tissue distribution characteristics or restenosis inhibition properties as compared to sirolimus itself. The new limus agents-everolimus, tacrolimus and ABT-578-all bind to the protein FKBP12 and have the ability to inhibit smooth muscle cell proliferation. However, there is controversial data regarding their clinical efficacy.
Three small efficacy trials (FUTURE I and II, SPIRIT FIRST trials) evaluating everolimus-eluting stents demonstrated effective reduction of neointimal proliferation at 6 months (in-stent late loss 0.10 mm in FUTURE I, 0.12 mm in FUTURE II, and 0.10 mm in SPIRIT FIRST) as well as equivalent or better MACE when compared to bare metal stents10-13. However, 2 small non-randomised trials of tacrolimus-eluting stents showed suboptimal results. The high in-stent late loss of 0.81 mm in PRESENT I and the 32% MACE rate in PRESENT II were unsatisfactory, which was believed to be related to the stent coating14.
Although no head-to-head data are yet available, the in-stent late loss of 0.26 mm of this Biolimus A9™-eluting stent compares favorably with that of sirolimus-eluting Cypher stents (-0.01 to 0.20 mm)3-5 and paclitaxel-eluting Taxus stents (0.30 to 0.39 mm)7,8,15,16. This efficacy is accompanied by a safety profile similar to that of the bare metal control stent. The MACE rates (3.8% in Biolimus stent group vs. 2.5% in S-Stent group) were remarkably low in both groups.
In addition to what appears to be a potent limus drug (BA9), the presence of the new asymmetric bioabsorbable PLA polymer coating on the study stent may prove to be an advantage, as durable polymers are believed to be a permanent source for inflammatory reactions that may lead to restenosis after the drug is completely eluted. The PLA polymer used in STEALTH I is completely resorbed by 6 months, leaving only the bare metal stent and thereby reducing the risk of any late adverse events. The continued follow-up of STEALTH I will provide further insights into these long-term effects.
Study limitations
This study is a small, three-center study that is not powered as a superiority trial for clinical outcomes. The excellent performance of the bare metal S-stent group (6-month TLR 0%, MACE 2.5%, and in-stent binary restenosis 7.7%) in this patient group with relatively simple lesion characteristics also makes it more difficult to discern significant differences with the BioMATRIX™-Stent group.
Conclusion
This pilot trial demonstrates that the bioabsorbable-polymer-coated Biolimus A9™-eluting BioMATRIX™ stent safely and effectively reduces neointimal proliferation and restenosis when used to treat de novo coronary lesions.

