This study was partially supported by “The Medicines Company”
Abstract
Objective: This open label, prospective, non-comparative trial is the first to evaluate the safety and feasibility of bivalirudin (Angiomax®, the Medicines Company, Parsippany, NJ), during PCI with implantation of the sirolimus eluting stent (Cypher, Cordis a J & J, Warren, NJ) or the paclitaxel eluting stent (Taxus, Boston Scientific, Natick, MA).
Methods: Patients who were referred for elective PCI suitable for stent implantation were recruited. Bivalirudin was administrated as a bolus of 1.0 mg/kg followed by 2.5 mg/kg/hour infusion for up to 4 hours in the first 68 patients. Following REPLACE 2 study, patients were treated with a lower dose of bivalirudin (0.75 mg/kg followed by 1.75 mg/kg/hour infusion). Results: 111 patients with 139 lesions were included in the study. Thirty-one (27.9%) were diabetics, 14 (12.6%) had unstable angina and 79 (66.6%) had multivessel disease. Complex lesion morphology was present in 65 (46.7%), in-stent restenosis in 32 (23%), total occlusion 7 (5%) and bifurcations 22 (15.8%). Activated clotting time (ACT) was verified to be therapeutic following bivalirudin administration. During the index procedure no patients required provisional use of glycoprotein (GP) IIb/IIIa inhibitors. One patient had intracoronary thrombosis which resolved after stenting. An additional 2 patients had non-Q wave myocardial infarction (MI). No patients died, had major bleeding, required transfusions or sustained vascular complications. 1 patient developed thrombosis at 4 months with a documented MI. At 6 month follow-up, 3 (2.7%) patients died and 12 (10.8%) patients had target lesion revascularization (1 CABG and 11 re-PCI). Angiographic follow-up was achieved in 98 patients (89.9%). Conclusions: This study indicates the safety and feasibility of Cypher or Taxus stent implantation in conjunction with bivalirudin administration, with no elective use of GP IIb/IIIa inhibitors.
Recent trials have demonstrated the benefit of drug-eluting stents for the prevention of restenosis after percutaneous coronary intervention (PCI)1-4. Previous clinical experience shows that bivalirudin (Angiomax, The Medicines Company, Parsippany, NJ), a reversible short-acting thrombin inhibitor, can be used in conjunction with uncoated stents5-7. Recently a retrospective, observational study evaluated the efficacy and safety of bivalirudin compared with heparin in patients treated with sirolimus-eluting stents (SES) (Cypher, Cordis a J & J, Warren, NJ)8. Despite extensive and increasing usage of this drug in the USA, in Europe bivalirudin is currently commercially available only in Denmark, Sweden, Finland, Germany, Austria and United Kingdom.
This open label, non-comparative, prospective trial is the first to evaluate the safety and feasibility of bivalirudin during PCI with placement of either SES or paclitaxel-eluting stent (PES) (Taxus, Boston Scientific, Natick, MA).
Methods
From February 2003 to January 2004, all consecutive patients referred to San Raffaele Hospital for elective PCI, with at least one coronary lesion requiring the placement of a SES or PES, were included in the study. Initially all patients enrolled were treated with SES implantation and following the availability of PES this stent was used consecutively.
The research protocol has been approved by San Raffaele Hospital Ethic Committee and informed consent has been obtained from all subjects enrolled in the study.
Coronary angioplasty and drug-eluting stent (DES) implantation was performed according to the practice of fully covering the diseased segment2-4,9. Bivalirudin was administrated during the procedure as a bolus of 1.0 mg/kg followed by 2.5 mg/kg/hour infusion for up to 4 hours in the first 68 patients. Following “The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events” (REPLACE)-2 trial7 patients were treated with a lower dose of bivalirudin (0.75 mg/kg followed by 1.75 mg/kg/hour infusion). In all patients activated clotting time (ACT) was performed at baseline, 5 minutes after the bolus (if ACT<250 sec a re-bolus of 0.5 mg/kg was done) and at the end of the procedure. Glycoprotein (GP) IIb/IIIa inhibitors were administered on a provisional or unplanned basis.
Patients commenced combination antiplatelet therapy of aspirin (at least 100 mg qd) and ticlopidine 250 mg bid or clopidogrel 75 mg qd at least 3 days prior to the procedure which continued for at least 6 months. A loading dose of 300 mg of clopidogrel was administered to those not previously taking thienopyridines.
Coronary angiograms were analyzed using a semi automated edge contour detection computer analysis system (MEDIS QCA CMS, version 4)10 at baseline, after the procedure and at follow-up.
At baseline and after 24 hours full blood count with white blood count was performed. At baseline, after 8-12 hours and after 24 hours from the index procedure, cardiac enzymes (total CK and CK-MB) were measured.
The occurrence of death, MI, revascularization and haemorrhage was recorded from enrolment through hospital discharge, at 1 month clinical follow-up and 6 months clinical and angiographic follow-up was scheduled in all patients.
Definitions
The primary end-points of the study were: death from all causes, myocardial infarction (MI) either Q wave or non-Q wave, revascularization (target lesion, target vessel or any revascularization) and bleeding (combined TIMI criteria) which occurred during the hospitalisation.
Secondary end-points were: death, MI, target lesion revascularization (TLR), and target vessel revascularization (TVR) which occurred during post discharge follow up.
Deaths were classified as either cardiac or non-cardiac. Deaths that could not be classified were considered to be cardiac. Non-Q wave MI was defined as an elevation of CK-MB 3 times above the upper limit of normal (ULN), in the absence of pathological Q waves.
Haemorrhage was classified based on the guidelines defined in the TIMI trial11.
Restenosis was defined as >50% luminal narrowing in the treated segment (stent and 5 mm proximal and distal) demonstrated at the follow-up angiography, irrespective of clinical symptoms of the patient.
TLR was defined as any revascularization performed on the treated segment; TVR was defined as any re-intervention performed on the vessel treated. Major cardiac events (MACE) were evaluated as the occurrence of cardiac death, MI and revascularization of the target lesion or the target vessel. Cumulative MACE was considered as the cumulative occurrence of MACE in-hospital and during follow-up.
Statistical analysis
Data are presented as percentages and as means ± SD.
Differences in proportions were tested with χ2 test or Fisher’s exact test, while differences in location parameters of continuous variables were tested with student’s test. The univariate and multivariable relationship of TLR as well as of restenosis to covariates were assessed with logistic regression. Results are reported as odd ratio (OR) with 95% Confidence Interval (CI). Analysis was performed using Stat View (SAS Institute Inc, Cary, NC).
Results
One hundred-eleven patients were included in the study: mean age was 60.0 ± 10.7 years, 31 (27.9%) were diabetics, 14 (12.6%) had unstable angina and the ejection fraction was 54.3 ± 9.2%.
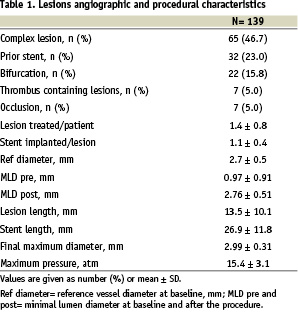
DES were implanted in 139 lesions. Angiographic and procedural characteristics of the lesions are summarized in Table 1. Of these 65 (46.7%) were complex lesions (B2 and C lesions according to AHA/ACC modified classification)12, 32 (23%) were in-stent restenosis, 7 (5%) chronic total occlusions, 22 (15.8%) located at bifurcations and 7 (5%) thrombus-containing lesions. Reference vessel diameter was 2.7 ± 0.5 mm and lesion length 13.5 ± 10.1 mm. The mean number of lesions per patient was 1.4 ± 0.8 mm. Seventy patients were treated with SES and 41 with PES. Stent length per lesion was 26.9 ± 11.8 mm and maximum balloon pressure was 15.4 ± 3.1 atm.
The ACT at baseline was 129 ± 26 sec and it was maintained at optimal therapeutic levels throughout the procedure (ACT post-bolus = 397 ± 67sec and at the end of the procedure = 352 ± 43 sec). Mean time of bivalirudin infusion was 92.3 ± 104.7 minutes; 78 (69.3%) of the patients interrupted bivalirudin infusion after the procedure. During the index procedure no patient required provisional use of GP IIb/IIIa inhibitors; 1 patient had coronary perforation managed by discontinuation of bivalirudin infusion. One patient had intracoronary thrombosis during the index procedure which resolved after stenting. During hospitalisation the same patient also developed sub-acute stent thrombosis (SAT) and a non Q wave myocardial infarction treated with repeat stent implantation and tirofiban infusion (this patient had a partial deficit of protein S). An additional 2 patients had a non-Q post-procedural MI. No patient died, had major or minor bleeding according to our study definition, required transfusions or sustained vascular complications. Only 1 patient had gum bleeding prompted resolved after drug discontinuation without experience any other adverse event.
Follow-up events
One patient treated with two Taxus stents, utilising the T technique, in a left anterior descending diagonal bifurcation, presented with thrombosis in the diagonal branch and consequent antero-lateral non Q wave MI. This occurred at 4 months while the patient was on double antiplatelet therapy.
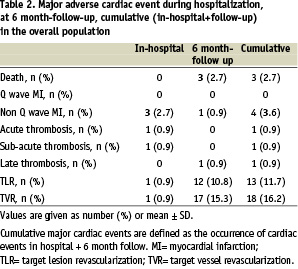
At 6 ± 1 month-clinical follow-up, 3 (2.7%) patients had died. Two patients with low ejection fraction died of sudden death, 1 patient died after CABG. Twelve (10.8%) patients had TLR and 17 (15.3%) had TVR. Only 1 patient underwent CABG (Table 2).
Angiographic follow-up was performed in 89.9% of the pts. Restenosis occurred in 14 lesions (11.6%). Two patients had occlusive in-stent restenosis (1 patient in Cypher and the other patient in Taxus), in all other patients restenosis was focal. Late loss was 0.31 ± 0.76mm and loss index 0.15 ± 0.39 (Table 3).
Lesion length showed a trend as the only predictor of TLR (OR= 1.059; CI= 0.99 to 1.131; p= 0.08) and restenosis (OR= 1.049; CI= 0.991 to 1.111; p= 0.09) on multivariate analysis.
Discussion
The main findings of this study are: -use of bivalirudin as antithrombotic agent for patients undergoing SES or PES implantation is clinically feasible and safe with an extremely low rate of non Q wave MI (2.7%) – bivalirudin can be used as a single antithrombotic agent during DES implantation (no patient required provisional treatment with GP IIbIIIa inhibitors) – despite the moderate risk profile of the study population, the re-PCI rate is acceptable.
Several clinical studies have shown that bivalirudin, a reversible short-acting thrombin inhibitor, was a safe and effective antithrombotic agent when used in conjunction with uncoated stents6,7,13-15. Moreover, it has been reported that bivalirudin had lower rates of ischemic and bleeding complications when compared to unfractioned heparin.6,7,14,15 Since the market release of SES and PES, several issues have been raised regarding their potential thrombogenicity despite low thrombosis rates reported in clinical trials1-4,16,17. The occurrence of some episodes of intra-procedural stent thrombosis, in an early phase of our experience, has prompted us to become more aggressive with the use of GP IIb/IIIa inhibitors and to strictly aim for an ACT value above 250 sec18. Recently, a retrospective study has evaluated the treatment of bivalirudin compared to unfraction heparin when SES was implanted8.
Our registry was a prospective, open label, non-comparative trial, the first to evaluate the safety and feasibility of bivalirudin during PCI with placement of either SES or PES. At the beginning of our experience a higher dose of the bivalirudin was adopted, but after the results of REPLACE 2 a lower dose regimen was chosen: no significant difference in the the occurrence of in-hospital MACE was present. Regarding the issue of acute (AT) and sub-acute stent thrombosis (SAT), in our study only 1 patient had intracoronary thrombosis during the index procedure which resolved following stent implantation. The same patient had a SAT treated with re-PCI. Hematologic work-up showed that this patient had a partial deficit of protein S, a situation which predisposes to thrombosis. No other patients had AT or SAT. The rate of either AT or SAT was 0.9% which is comparable with the reported data of PES and SES in conjunction with heparin in clinical trials as well as in “real world” subset1-4,16. The rate of non Q wave MI (2.7%) is very encouraging taking in consideration the lesion and patient characteristics and the incidence of periprocedural MI reported in the literature8,19-22. Moreover, no patient had a Q wave MI and no patient died during the hospitalization. One patient had intra-coronary perforation managed with bivalirudin cessation without cardiac tamponade. No patient had major or minor bleeding and no patient required transfusion. The low occurrence of in-hospital events without any use of provisional GP IIbIIIa inhibitors provides further assurance of the safety and efficacy of continued use of bivalirudin as a single antithrombotic with DES. The fact that no patient needed an extra bolus of bivalirudin confirms that ACT is reproducible when bivalirudin is used during PCI and does not correlate with clinical events23. Further information regarding the safety of the use of bivalirudin and DES in the setting of ST elevation myocardial infarction (STEMI) will come from the “Harmonizing Outcomes with Revascularization and stents” (HORIZONS) trial. In the HORIZONS trial 3400 patients will be treated with either TAXUS or BMS and additionally randomized to a combination of unfractionated heparin and glycoprotein (GP) IIb/IIIa inhibitors or bivalirudin ± bailout GP IIbIIIa inhibitors. There will be a 5 year clinical follow-up period and 1,500 patients will undergo angiographic follow-up.
Despite the moderate-high risk profile, testified by clinical, lesion and angiographic characteristics of the study population (in-stent restenosis, total occlusions and bifurcation lesions were included), the occurrence of TLR (10.8%) as well as TVR (15.3%) was reasonable and can also be explained by the high rate of angiographic follow-up (89.9%). The high rate of angiographic follow-up could have prompted to the increased usage of revascularization when many of these focal restenotic lesions were not associated with angina and few patients had non-invasive testing in search of for ischemia. The general reluctance to leave a focal angiographic narrowing of a major epicardial vessel is a frequent preoccupation, which may justify an “oculostenotic reflex”.
Conclusions
No specific interactions between bivalirudin, SES or PES were observed. This study indicates that SES or PES implantation may be safely performed in conjunction with bivalirudin administration, with no elective use of GP IIb/IIIa inhibitors.

