Abstract
Aims: To answer the question whether the delay in coronary artery bypass grafting (CABG) after carotid stenting (CAS) results in adverse events, we describe our experience with planned staged CAS followed by CABG.
Methods and results: We retrospectively surveyed our hospital carotid stenting data base, to identify all patients who were scheduled to undergo staged carotid stenting followed by CABG. A total of 39 patients who underwent CAS were candidates for staged CABG but only 28 (71.7%) of them referred. In the interval between carotid stenting and CABG, 4 deaths occurred (14.2% of cases), all of them were in the first week after CAS and due to cardiac problems. Also, 2 patients (7.1%) had a minor stroke. Increased number of predictors of type C (most important was stenosis of 95%-99%), age > 75 or significant valvular heart disease were associated with increased rate of complications after CAS.
Conclusion: Should carotid intervention be performed in the high risk group of patients with > 4 suggested predictors of type C (most importantly is stenosis of 95%-99%), valvular heart disease or age > 75, physicians should closely observe the patients (perhaps in the hospital) during the waiting period before CABG, particularly in the first week after carotid stenting.
Introduction
The coexistence of coronary and extracranial carotid atherosclerotic disease is 1.7%-12%1 and a carotid stenosis of >70% is identified in 8% of patients evaluated for coronary artery bypass surgery (CABG)2. Guidelines for the treatment of coexistent coronary and carotid disease especially for the proportion of patients who require CABG, do not exist and practices vary greatly1. In patients who require CABG, the risk of perioperative stroke is 4 fold higher for those with a past history of TIA or stroke and 10 fold higher in asymptomatic patients with carotid stenosis greater than 75%3.
Surgical approaches for simultaneous CABG and carotid revascularisation have included staged separate procedures (either CABG followed by carotid endarterectomy [CEA] or vice versa) or synchronous CABG and CEA. A systematic review of outcomes following staged or synchronous CEA and CABG showed that 10-12% of patients who were reported as case series from tertiary centres of excellence, suffered death, stroke or myocardial infarction within 30 days of surgery4.
A recently published review of a community based study in the US reported that after correcting for comorbidities, the odds ratio for postoperative stroke or death for CEA-CABG was 1.38 vs CABG5.
Carotid artery stenting (CAS) has been explored as a less invasive approach compared to CEA for the treatment of atherosclerotic disease in the setting of severe coronary artery disease6.
One randomised study and several registries7,8 have now reported that patients at high operative risk for CEA have better outcomes when treated with carotid stents. But the place for carotid intervention in asymptomatic carotid stenosis routinely before CABG remains controversial, particularly because it is still unclear what additional workload carotid intervention before CABG would place on overstretched vascular services9. Since CAS patients are treated with clopidogrel for one month, it is best to defer CABG for 5 weeks3.
Very little data are available in the literature describing carotid stenting followed by staged CABG1. To answer the question whether the delay in CABG results in adverse events, and the risk factors of these adverse events; we describe our experience with planned staged carotid stenting followed by CABG in a specialised large volume heart hospital.
Patients and methods
We retrospectively surveyed our hospital carotid stenting data base, to identify all patients who were scheduled to undergo staged carotid stenting followed by CABG. This survey explored the intention to treat basis, so that we also recognised the patients who were candidates to undergo both procedures, but didn’t do so for any reason. After written informed consent was obtained, symptomatic patients with more than 60% stenosis and asymptomatic patients with more than 80% stenosis of either internal or common carotid artery were included in the study. Patients were excluded if any of the following were applicable: patients with unstable cardiac condition, age > 85 years, history of major stroke within the last week, pregnancy, intracranial tumour or arteriovenous malformation, severely disabled as a result of stroke or dementia and intracranial stenosis that exceeded the severity of the extra cranial stenosis. Eligibility for CABG was at the discretion of the responsible cardiologist or the treating surgeon based on standard ACC/AHA guidelines. Eligibility for CAS was determined based on the joint decision of the interventional cardiologist, cardiac surgeon and neurologist.
All patients underwent formal neurological and cardiac assessment on hospital admission. Patients received a combination of clopidogrel 75 mg and aspirin 325 mg for 5 days before carotid stenting or, alternatively, loading doses of clopidogrel (600 mg) and aspirin (650 mg) at least 4 hours before the procedure. On the day of the procedure, oral antihypertensive therapy was withheld, and adequate volume status was ensured. The technical details of the procedure have been previously reported10. However, aggressive volume expansion, intravenous dopamine, and occasionally dopamine infusions were sometimes necessary. Blood pressure elevation after the relief of the stenosis was treated with intravenous nitroglycerine.
Patients were followed up in a monitored environment by staff familiar with the post procedural course and with groin access site management. If the neurological examination was unchanged and the procedure was uncomplicated, patients were discharged 2 days after the procedure on a regimen that included ASA and clopidogrel for 4 weeks.
Data collection and endpoints
A complete neurological history was taken and an examination performed on all patients by an experienced neurologist. The study protocol required that an independent neurologist not involved in the interventional procedure evaluate patients using the NIH Stroke Scale11 before the procedure and at 24 hours, 1 month and 6 months after the procedure. Before the procedure, Carotid duplex study, MRI or computed tomography of the head and complete diagnostic angiography, including intracranial views and assessment of the collateral circulation, were performed on all patients. All clinical, angiographic and stenting data were prospectively recorded on standard forms by a physician. Clinical and laboratory details were continuously recorded during the hospitalisation. The clinical end points were: ipsilateral transient ischaemic attack (TIA) and any ipsilateral major or minor stroke, myocardial infarction or death during follow up period. Long-term follow-up was then conducted by telephone interviews or clinical visits for all patients who were presented for CABG. Clinical end points were critically reviewed by the independent neurological members of the study group. If a patient had neurological deterioration after stenting, MRI or computed tomography of the head was repeated.
A peri-procedural stroke was defined as any new neurological deficit that persisted more than 24 hours. A major non-fatal stroke was defined as a new neurological deficit that persisted > 30 days and increased the NIH (National Institute of Health) stroke scale by > 4. The remaining strokes were classified as minor which was defined as a new neurological deficit that either resolved completely within 30 days or increased the NIH stroke scale by < 3. Neurological deficits that fully resolved within 24 hours were classified as a transient ischaemic attack (TIA)12.
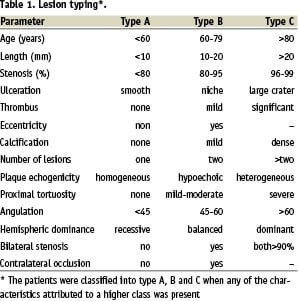
Morphological data, lesion characteristics (eccentric, calcified, ulcerated, multiple) and type of lesion (A, B and C)12 were recorded retrospectively by reviewing the angiographic films. Any of the characteristics in class C, is considered as a predictor for events (Table 1).
All values are expressed as mean ± SD. Categorical variables are expressed as percentages. Results have been described and analysed on an intention-to-treat basis except where otherwise stated.
Results
From January 2004 through February 2006, a total of 39 cases (20 males, 19 female) were scheduled to undergo carotid stenting followed by scheduled CABG. Subsequent to successful carotid stenting, 11 patients elected not to undergo CABG; none of them suffered a persistent adverse event in the follow-up period. A total of 28 presented for CABG. The demographic and clinical characteristics of these patients are shown in Table 2.
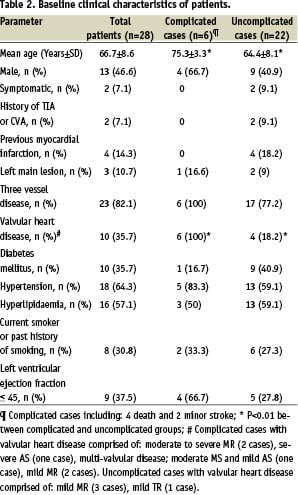
Our patients were predominantly grade 3 or 4 on the Mayo Clinic carotid endarterectomy risk classification scheme13. The patients had significant comorbidity and all were considered to be high operative risk for CEA. Many of them had 3 vessel coronary artery disease (82%) and valvular heart disease (36%). Technical and angiographic success was achieved in all 28 patients. Carotid artery stenosis diminished from 89.25±8.6% to 15.2%±3.5% (by careful visual estimate at angiography). Twenty-eight stents were implanted in the 28 vessels: 13 (46.4%) Acculink (Guidant, Santa Clara, CA, USA), 15 (53.6%) Wallstent (Boston Scientific Corp., Watertown, MA, USA). Complication rates were not different between these stents. Protective devices used in all cases included: 23 (82.1%) EZ (Boston Scientific-EPI, Santa Clara, CA, USA) and 5 (17.9%) AccuNet (Guidant, Indianapolis, IN, USA). In all patients, post-dilation was done. The mean vessel diameter was 7.32±1.09 mm and the mean diameter of the post-dilation balloon was 5.48±0.55 mm. The mean post-stenting balloon inflation pressure was 8±2.08 atm. After stent deployment, control cerebral angiogram was normal in all patients. Neurological assessment showed transient neurological deficit in one patient during the procedure.
Clinical outcome and follow-up
The mean interval between carotid stenting to CABG was 78.8±60 days (median 80). Twenty-eight patients presented for CABG. This interval was at the discretion of the treating cardiologist or cardiac surgeon and dictated by the severity of symptoms and the extent of coronary atherosclerosis. Where possible, patients did not undergo CABG within 4 weeks of surgery. Due to refractory unstable angina one patient underwent CABG 8 days after CAS. Except from that patient; clopidogrel was withdrawn in all others, > 5 days prior to the cardiac surgery.
There were 4 deaths (the first was 12 hours after CAS due to cardiovascular collapse and asystole because of MI, the other case died in the CCU after 3 days with pulmonary oedema and cardiogenic shock, another died 5 days after discharge with MI and the last was 4 days after procedure in CCU with MI) and 2 minor strokes (one had transient cognitive disorder and the other had conductive aphasia due to ischaemic stroke, both of them recovered to baseline within 7 days) in the interval between carotid stenting and CABG. Therefore, our overall rate of complication and mortality rate in interval period was 21.4% and 14.2% respectively (Figure 1).
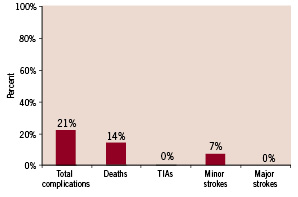
Figure 1. Death and complication rate in interval period between carotid stenting to CABG (n=28).
As shown in Figure 2, all mortality occurred in the first week after CAS.
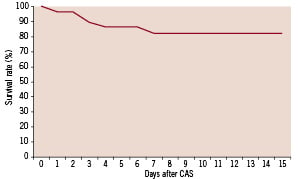
Figure 2. Survival rate in interval between CAS and CABG.
Long-term clinical follow-up was possible for all patients. After CABG the mean follow-up period was 11.3±6.1 months (15 days - 20 months). During this period, one cardiac death occurred the first day after CABG due to electro mechanical dissociation. There was also one patient who presented with one episode of TIA within 7 days of discharge after CABG.
Predictors of events
Clinical characteristics of patients with and without complications are shown in Table 2. Based on previous studies, the patients were classified into type A, B and C when any of the characteristics attributed to a higher class were present10. Guidelines for stratifying patients on class C are proposed in Table 3.
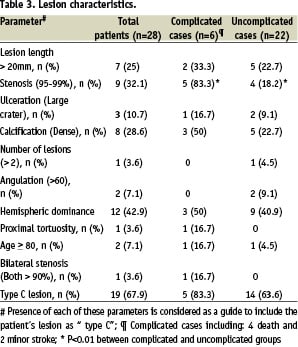
There were significant differences between complicated and uncomplicated cases in mean age (p=0.004), valvular heart disease (p=0.001) and stenosis of 95-99% (p=0.003). As seen in Figure 3 and 4, increased numbers of including criteria of class C are associated with increased rates of complications after the CAS. Also, between these predictors stenosis of 95% - 99% is more important than the others. In complicated cases, 83.3% had stenosis of 95%-99% (Table 3).
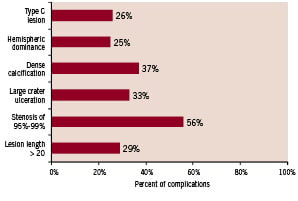
Figure 3. Complication rate in different lesion characteristics.
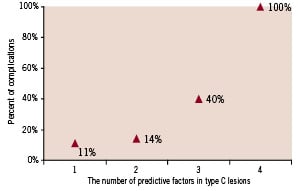
Figure 4. The relationship between complication rate and the number of predictive factors in type C lesions.
Discussion
Data obtained from tertiary centres suggests that 10-12% of patients undergoing staged or simultaneous CEA and CABG suffered death or major cardiovascular morbidity (stroke, MI) within 30 days of surgery2,13. A review of a U.S. community data base and some other isolated reports of patients who underwent simultaneous CEA & CABG show adverse event rates of about 18% and even more8.
Due to the results of large studies such as SAPPHIRE trial, ARCHER, and SECURITY, carotid stenting has been considered as the standard-of-care in patients who have high operative risk factors for CEA8,14,15. Thirty-day major adverse event (MAE) rates (death, any stoke or MI) in these trials are between 5.3 and 7.8%14-18 compared to reported MAE rate of up to 12.6% in high risk patients undergoing CEA.
This study is one of the few studies to critically report the neurological and clinical complications of carotid artery stenting in high risk patients with severe coronary artery disease who are candidates for open heart surgery. These findings, based on previous lesion typing studies12, suggest a new evaluation system which considers any one of the characteristics in class C, particularly stenosis of 95% - 99% as a predictor for major events10. Increased number of these predictors is associated with increased rate of complications after the CAS. Furthermore increasing lesion severity (> 95% stenosis) was found to have the most association with increased incidence of complications. This finding is comparable to other study which has been shown the risk of stroke in the territory of an asymptomatic carotid stenosis was strongly dependent on angiographic lesion severity.19
There was no major disabling stroke. Minor non-disabling stroke occurred in 7.1% of vessels treated, which represented a rapidly resolving minimal cognitive disorder and conductive aphasia that was probably due to small amounts of embolic debris. Comparing the results with previous endarterectomy or stenting series, the stroke rate seems to be the same14,17,20,21.
There was no clinical or para-clinical clue in favour of a neurologic sequel as the primary cause of mortality in expired patients. High mortality (14.2%) observed, in our study might be explained by the clinical characteristics of the population, with 100% of the patients suffering from coronary artery disease.
To our knowledge, there are very few studies on the use of carotid stenting in the management of concomitant severe carotid and cardiac disease. Randall et al9 assessed outcomes of staged CAS and CABG in 52 patients. All of their patients had coronary artery disease and were candidate for CABG which were the same as ours. At that study 5.7% of their patients had died in the waiting period before the CABG. In contrast to our results, all of them occurred between 20-60 days after CAS and none of them were stroke related death. As their report shows, they used less often distal protection devices (69%) or dual antiplatelet therapy (83%) for their patients. The prevalence of valvular heart disease, LV dysfunction, extent of coronary artery disease and carotid lesion characteristics has not been mentioned. Ziada et al22 compared the outcomes of 56 patients who underwent carotid stenting (CAS) followed by open heart surgery (OHS) with the outcomes of those who underwent combined CEA + OHS. Compared with our study, the CAS + OHS group in their study included similar percentage of patients with severe angina and severe coronary disease, 37% of their patients underwent complex OHS which included repeat OHS and/or OHS involving valvular and/or aortic surgery in addition to CABG. Compared to our study, the time between CAS and OHS was less (39+22 days median 40 compared to 78+60 days median 80). In their study, the reported stroke rate was 1.8%, although they did not mention whether the stroke was minor or major (in our study there was no major stroke and the rate of minor stroke was 7%). Compared to 14.2% mortality rate in our study, 5.4% of their patients died after CAS and before OHS, all of them had severe 3 vessel disease. There is no data whether or not those patients had significant valvular heart disease.
Kovacic et al1 recently described 20 patients who underwent carotid stenting followed by CABG. They reported 5% myocardial infarction and 5% minor stroke, no cardiac death and major stroke has reported. On the contrary, our study showed more death and other major complications. All the mortality rate occurred early after stenting (in the first week) and all of the patients who died had significant valvular heart disease. Considering the above mentioned observations, it seems that the deaths were due to profound hypotension and cardiovascular collapse, most likely initiated by carotid baroreceptor changes after stenting. It may be due to the fact that in our study there were more patients with significant valvular heart disease and multivessel disease (35.8% versus 8.7% and 100% versus 95.7%, respectively). The interesting finding of high incidence of complications after CAS in patients with valvular heart disease might be due to the more severe haemodynamic changes after stenting in this particular group of patients. It was recently reported that patients with persistent haemodynamic depression were at a significantly higher risk of developing a MACE (OR=3.05) or stroke (OR=3.34)23. One group reported that patients with large fluctuations in SBP after CAS were at a significantly higher risk of stroke, whereas patients with smaller fluctuations did not experience an adverse neurologic event24. Another group found that patients with hypotension were at increased risk of in-hospital minor strokes and death at six months when compared with patients who did not have hypotension25. In another study, Lopes et al26 presented their experience with CAS in 49 patients with concomitant carotid artery and coronary artery disease. They showed 8% cardiac death, 2% major stroke, 6% minor stroke and a combined incidence of complications of at least 16%. Furthermore, two out of four deaths occurred within 24 hours of CAS, one in the interval period before CABG and another during CABG. To avoid more deaths in the interval between stenting and CABG they performed CABG as soon as possible without interrupting antiplatelet therapy. Although reasonable in a patient with unstable coronary condition, it seems that bleeding complications may out weigh the benefits of early CABG in stable patients. Comparing with the study of Lopes et al26, patients in our study underwent CABG at least 8 days after CAS (mean duration = 78.8±60) and most of them stopped receiving antiplatelet therapy at least 5 days before CABG. Also, the fact that all mortality occurred in the first week after CAS shows that there might be no significant benefit (and actually an increased risk of hazards due to the increased rate of bleeding) in performing CABG as early as the second week after CAS, instead of waiting for more than one month to complete the course of clopidogrel treatment.
Two recently published European randomised studies comparing CAS with CEA27-29 showed much higher complication rate of CAS than studies which were conducted in the United States. Mas et al27 reported the 30-day incidence rate of any stroke or death of 9.6%. Their rate of minor stroke of 8.1% seems to be higher than ours (7.2%). They did not use distal protection devices and dual antiplatelet therapy in 10-15% of their patients. Also, the investigators of the SPACE trial were unable to prove the non-inferiority of carotid stenting with regard to endarterectomy.28 Both these trials involved patients who had an average risk of perioperative stroke or death after surgery; patients who had severe coronary artery or valvular heart disease and patients with severe angina pectoris were excluded.
Comparison of baseline characteristics in complicated and uncomplicated patients showed that all complicated patients were > 70 years old of age and had three vessel disease and valvular heart disease. Although all of these 3 factors are highly sensitive in predicting complications, their absence allows for sufficient specificity to rule out the probability of major complications.
Based on the above mentioned typing classification12, we considered any of the characteristics in class C as a predictor for events and found the total number of predictors as an invaluable index for prediction of major complications after carotid stenting. Indeed, increased number of predictors of type C (most important was stenosis of 95%-99%) in addition to age > 75 or having valvular heart disease was associated with increased rate of complications after CAS.
Study limitations
The primary limitation of this study is that the sample size was not large enough, so it lacks statistical power to adequately differentiate the relative risk of each variable. Validation of these predictors requires larger prospective studies. Also, this study cannot be comparable to reported trials and series of CEA because of confounding factors that may make patients at higher risk. This will ultimately be tested in randomised clinical trials.
Conclusions
This study carefully defined the demographic, clinical and lesion characteristics of patients undergoing carotid stenting in Tehran Heart Center and showed that the complications of carotid stenting are dependent on patient selection. On the basis of this suggested evaluation system, it appears that in the case of carotid intervention for patients with stable cardiac condition, but with > 4 suggested predictors of type C (most importantly being stenosis of 95%-99%), valvular heart disease or age > 75, physicians should closely observe the patients (perhaps in the hospital) during the waiting period before CABG particularly in the first week after carotid stenting.
To determine whether the best treatment strategy for this group of patients is carotid stenting with delayed coronary bypass surgery or a simultaneous endarterectomy and coronary bypass surgery, we need to begin a prospective randomised clinical trial. However, this study didn’t show any procedural complications with such patients, thus it seems that with appropriate patients selection and carotid care after the procedure; carotid artery stenting could be feasible and safe, even in patients who have significant coronary artery disease and are CABG candidates.
Acknowledgements
The authors would like to thank Ms. Kathryn Dougherty (peripheral vascular interventional research staff, Texas Heart Institute), Shahin Akhondzadeh PhD (Professor of Neuroscience, Tehran University of Medical Sciences), Javad Kojouri MD (Assistant Professor of Cardiology, Shiraz University of Medical Sciences), Mehrdad Rezaee MD (Associate Professor of Cardiology, Stanford University) and Nader Fallah Msc (Regular member of board, Department of Biostatistics, Shahed University, Tehran, Iran) for their assistance. Also, many thanks to the staff of the Research Department, Division of Cath Lab and Radiology department of Tehran Heart Center.
References

