Abstract
Background: Whether restenosis remains a major, and potentially fatal, complication after percutaneous intervention for left main coronary artery (LMCA) stenosis and whether routine surveillance angiography should be a necessary part of the follow-up of these patients in current drug eluting stent (DES) era is largely debatable.
Methods and results: Patients who underwent elective treatment of unprotected LMCA with DES in three referral centres in Europe were pooled as follows: i) 147 patients treated in Massy, between 12th August 2002 and 31st December 2004; ii) 107 patients, treated in Milan, San Raffaele Hospital and Columbus Clinic, between 16th April 2002 and 31st July 2004; iii) 86 patients treated at the Thoraxcenter, Rotterdam, between 16th April 2002 and 28th June 2004 leading to a total of 340 elective consecutive patients. The rate of in-hospital mortality was 0.6% (2/340). The out-of-hospital event rate in terms of cardiac death or myocardial infarction was 1.2% between discharge and 3 month, 0.6% between three and 6 months and 0.6% between 6 months and 1 year. Two (0.6%) sudden and unexpected deaths were cumulatively observed, at 8 days and 2 months after intervention, respectively. The rate of confirmed or possible stent thrombosis was 0.9%. At 1 year, the out-of-hospital cumulative incidence of cardiac death or MI was 2.4%. In the cohort of patients who refused to undergo angiographic surveillance, no death and no MI occurred.
Conclusions: Cardiac and sudden death and the incidence of stent thrombosis after LMCA intervention with DES were reasonably low and compared favourably with what reported in non-LMCA lesions. At the time intimal hyperplasia is expected to peak (i.e. beyond 6 months), no increase of adverse events, in terms of death or myocardial infarction was observed.
Introduction
Following the widespread adoption of drug eluting stents in current practice, and their growing use in off-label indications, there is clear need to re-evaluate some of the dogmas on the treatment of the left main coronary artery (LMCA) stenosis when undertaken percutaneously.
In particular, whether restenosis remains a major, and potentially fatal, complication after LMCA treatment and whether routine surveillance angiography is needed to decrease the risk of cardiac and sudden death related to intimal hyperplasia is largely debatable and controversial.
In the ULTIMA registry, two hundred seventy-nine consecutive patients who had elective or emergent LMCA treatment with PCI at 1 of 25 sites between 1993 and 1998 were studied1,2. Forty-six percent of these patients were deemed inoperable or at high surgical risk. Thirty-eight patients (13.7%) died in hospital. The 1-year incidence was 24.2% for all-cause mortality and 20.2% for cardiac mortality. An excess of deaths was observed predominantly in patients initially deemed inoperable. However, 2% per month death rate among hospital survivors was noted over the first 6 months. Of concern, most of the myocardial infarction (MI) cumulatively observed at one year, occurred in the first three months after treatment. Based on these findings, the interpretation that restenosis may lead to major fatal or non fatal complications in this cohort of patients was put forward, and the need to perform routine surveillance angiography at 2 and 4 months after treatment was subsequently formulated. This recommendation was in agreement with previous studies showing that intimal hyperplasia after bare metal stenting peaks 12-16 weeks after intervention.
However, the uptake of this recommendation varied considerably between centres and, even when this recommendation has been followed, a single angiographic follow-up at 6 months, not two at 2 and 4 months, has been mainly carried out3-6.
The discrepancy between what has been recommended and clinical practice has several potential explanations.
Firstly, the evidence on which the need to perform routine angiographic surveillance relies is rather weak. Whether the clustering of events in the few months after LMCA treatment is directly related to the development of restenosis remains speculative since no protocol-mandated angiographic follow-up was pre-specified in the ULTIMA registry. Moreover, almost 50% of the included population was deemed to be inoperable or at high surgical risk, which may, in itself, explain the high incidence of adverse events observed in the study population (mortality rate at 1 year was 79%, 24% and 3% in high, intermediate and low risk patients, respectively). No demonstration that the performance of routine early angiographic follow-up translates into a clinical benefit in this patient population exists. Finally, there is concern for the iatrogenic hazard related to multiple angiographic follow-ups in this cohort of patients.
Drug eluting stent (DES) implantation, by reducing the need for target vessel revascularisation (TVR) and angiographic restenosis, has been recently shown to favourably affect outcome compared to bare metal stents (BMS) in patients undergoing percutaneous left main (LM) intervention. Current evidence suggests that both the magnitude and the peak of intimal growth may markedly differ in patients treated with DES as compared to BMS, which may offer the unique opportunity to re-evaluate the temporal incidence of major adverse events in this cohort of DES-treated patients.
Against this background, the principal aim of the present survey was to accrue data regarding the rate and temporal distribution of cardiac and sudden death or out-of-hospital MI in patients undergoing unprotected and elective LMCA intervention in the DES era. These observations might help re-examining the notion that in-stent restenosis may lead to fatalities or myocardial necrosis in patients undergoing percutaneous LMCA intervention. The second exploratory objective was to collect information in terms of clinical outcome on patients who refuse to undergo angiographic follow-up after LMCA treatment. This information may be critical in assessing the need for routine angiographic surveillance in this cohort of patients.
Patients’ selection
Patients who underwent elective treatment of unprotected LMCA with DES in three referral centres in Europe were pooled as follows:
1) One hundred forty seven patients, treated in Massy (France), between 12th August 02 and 31st December 04, [12 (8%) treated with sirolimus-eluting stent (SES) and 135 (92%) receiving paclitaxel-eluting stent (PES)]. This cohort of patients had a mean (SD) clinical follow-up of 6.8±2.12 months. All patients were initially scheduled to undergo 6-month angiographic surveillance, which was actually performed in 65% of eligible patients. No data regarding this cohort of patients has been previously reported.
2) One hundred seven patients, treated in Milan (Italy), San Raffaele Hospital and Columbus Clinic, between 16th April 02 and 31st July 04 [55 (51%) treated with sirolimus-eluting stent (SES) and 52 (49%) receiving paclitaxel-eluting stent (PES)]. All eligible patients underwent 1-year clinical follow-up. All patients were scheduled to undergo angiographic surveillance at 6 months, which was performed in 85% of those eligible. Six month outcome of the first 85 patients here included has been previously reported5.
3) Eighty-six patients treated at the Thoraxcenter, Rotterdam (the Netherlands) between 16th April 2002 and 28th June 04 [36 (41%) treated with sirolimus-eluting stent (SES) and 50 (59%) receiving paclitaxel-eluting stent (PES)]. All eligible patients underwent 1-year clinical follow-up. For research purpose, all patients were initially scheduled to undergo angiographic follow-up, which was finally carried out in 76% of those eligible. A subset of this cohort of patients has also previously reported3.
Thus, from 16th April 2002 to December 31st 2004, a total of 340 consecutive patients were treated, in three referral European centres, exclusively with one or more DES in the LMCA as part of an elective revascularisation procedure and constitute the patients population of the present report.
Procedures and post-intervention medications
All interventions were performed according to current standard guidelines and the final interventional strategy, including the use of glycoprotein IIb/IIIa inhibitors and stenting techniques, was entirely left to the discretion of the operator, except for the stent utilisation. All patients were advised to maintain aspirin lifelong, while clopidogrel was prescribed for at least 6 months in patients treated in Rotterdam or Milan or 2 months only for those undergoing treatment in Massy.
Endpoint definitions
The primary outcome of interest was the occurrence of total and cardiac mortality.
All deaths were considered to be of cardiac origin unless a non-cardiac origin was established clinically or at autopsy. Myocardial infarction (MI) was diagnosed by a rise in the creatine kinase level to more than twice the upper normal limit with an increased creatine kinase-MB fraction. The cumulative rate of post-discharge MI will be presented for all included patients, while occurrence of in-hospital myocardial infarction is available for only those patients enrolled at the Thoraxcenter.
Results
Mortality rate
In-hospital
The rate of in-hospital mortality was 0.6% (2/340). Overall, two fatal episodes occurred. Patient number 1, affected by unstable angina following a recent MI (Braunwald class III C) presented with distal LMCA stenosis. The proximal and mid LAD and circumflex arteries were also diffusely diseased. This patient was refused by surgeons due to severe left ventricular dysfunction (left ventricular ejection fraction=25%) and underwent an elective intervention under a left ventricular assist device, the Tandem Heart (Figure 1). The procedure was successful, but during the positioning of the last stent in the
circumflex artery a dissection of ascending aorta occurred. The patient died immediately after surgery. Patient number 2 was a 90 years old female admitted for unstable angina (Figure 2). Coronary angiogram revealed severe triple vessel disease. The patient was considered too old for surgery. She had an uncomplicated and successful intervention, but died 2 days after the procedure for haemorrhagic shock due to a severe groin haematoma, despite blood transfusion.
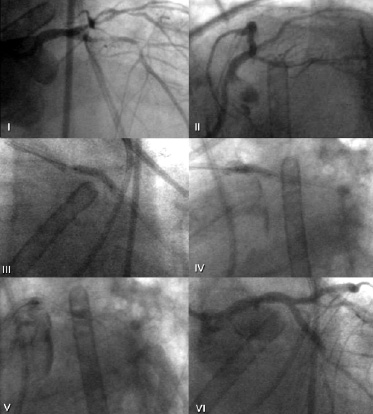
Figure 1. Index procedure in patient number 1, while assisted by left ventricular assist device, is shown before treatment (I and II), during stent implantation in the LMCA (III and IV) and at the end of the procedure (V and VI ) where dissection of the ascending aorta is visible.
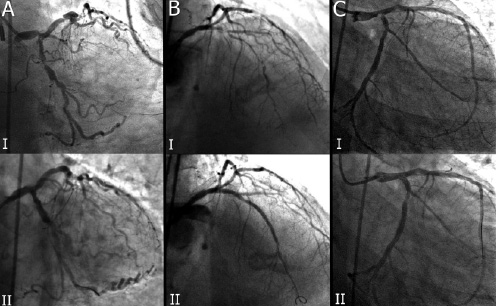
Figure 2. Index procedures of patients number 2 (A), number 3 (B) and number 9 (C) before (I) and after (II) treatment is shown.
Sudden death
Cumulatively, two sudden and unexpected deaths occurred (0.6%): i) patient number 3 died suddenly 8 days after the procedure; ii) patient number 4 (Figure 3) died 2 months after intervention after anti-platelet therapy discontinuation because of concomitant acute pancreatitis.
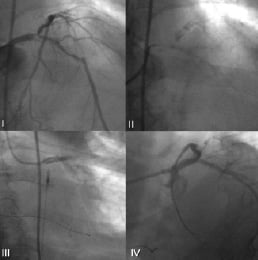
Figure 3. Angiogram of patient number 4 before treatment (I), showing diffuse coronary calcification (II), during intervention at the left main coronary artery (III) and immediately at the end of the procedure (IV).
Cumulatively total and cardiac mortality
There were a total of 13 fatal events (13/340, 3.8%), of which 8 (2.3%) were considered to be of cardiac origin as detailed in table 1. However, since patient number 2 died as a consequence of the index intervention (haemorrhagic shock due to groin haematoma), there were 9 (2.6%) cardiac or index procedure- related deaths (Figure 4, 5). In the remaining 4 patients, death occurred due to pulmonary infection (n=1), massive bleeding during dialysis (n=2) and ischaemic stroke (n=1).
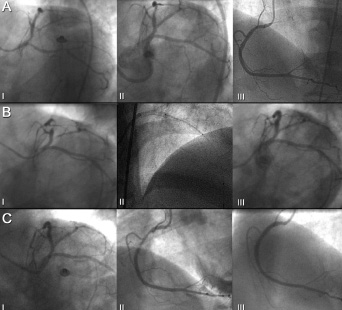
Figure 4. Patient number 6 at the time of the index procedure (Panel A) showing left coronary artery before (I) and after (II) treatment and right coronary artery (III). At first angiographic follow-up (Panel B) no restenosis of the left main coronary artery was detected (I). However, a significant in-stent restenosis of the proximal left anterior descending artery required reintervention (II) with excellent final angiographic result (III). At the second angiographic follow-up (Panel C), there was not detectable restenosis in the left coronary artery (I) while a progression of disease in the mid tract of the right coronary artery (II) required reintervention (III).
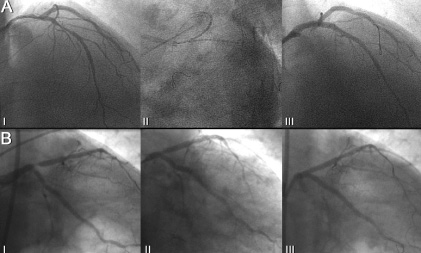
Figure 5. Patient number 7 during the index procedure (Panel A) before (I), during (II) and after treatment (III) and at the time of angiographic follow-up, showing a focal restenosis in the ostium of left anterior descending artery (II) which required reintervention with good final angiographic result (III).
Rate of stent thrombosis
One single episode of angiographically confirmed stent thrombosis (ACT) occurred (0.3%) 3 months after intervention in a patient who was still under double anti-platelet treatment. Hypothesising that the two observed episodes of sudden deaths were attributable to ACT, the rate of confirmed or possible stent thrombosis was 0.9%.
Rate of myocardial infarction
In-Hospital (n=84): There was no episode of Q-wave MI, while 4 (4/86; 4.6%) episodes of CK-MB elevation above twice the upper limit of normal (ULN) occurred. By elevating the threshold of CKMB rise to 3 times ULN, as currently recommended by ACC/AHA guidelines, the rate of MI was 1.1% (1/86). No episode of CK-MB elevation above 5 times ULN occurred.
Follow-up (n=340): There was one single episode of myocardial infarction which occurred at 3 month due to ACT, as previously described. Thus, the cumulatively rate of out-of-hospital MI at 1 year was 0.3%.
Cardiac death or myocardial infarction
The out-of-hospital event rate in terms of cardiac death or myocardial infarction was 1.2% between discharge and 3 month, 0.6% between three and 6 months and 0.6% between 6 months and 1 year. At 1 year, the out-of-hospital cumulative incidence of cardiac death or MI was 2.4%.
To explore the safety of avoiding systematic angiographic follow-up in this cohort, patients treated in Massy and Rotterdam were pooled, with a final population of 233 patients. Patients treated in Milan were not considered for this analysis due to the high rate of angiographic follow-up carried out in this cohort. Between discharge and 6 months, the rate of death was 3.4%, with no MI occurring after discharge. After 6 months, 160 (69%) patients underwent angiographic control. In this group, the rate of death from 6 to 12 months was 1.3%, with two cardiac deaths, both occurring due to complications during target vessel revascularisation (in one case during PCI, in the second case the patient died one day post-CABG) (Figure 6). In the cohort of patients who refused to undergo angiographic surveillance, no death and no MI occurred.
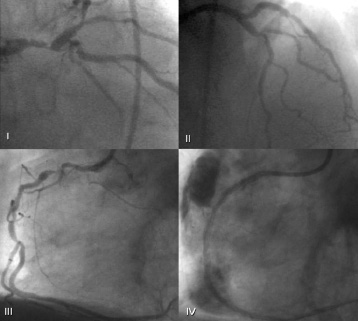
Figure 6. Patient 8 before (I) and immediately after (II) treatment. Due to persistence of symptoms, the patient had a second angiogram at follow-up showing a significant disease in the right coronary artery (III), during treatment a coronary rupture with pericardial effusion occurred (IV). The patient was sent to emergency surgery but died soon afterwards.
Discussion
With a total population of 340 patients, this report represents the biggest series of patients undergoing unprotected and elective LMCA intervention ever reported. In addition, by limiting our observations to only those patients undergoing DES-supported intervention, the current survey may represent a benchmark study for future randomised trials.
The main findings of this survey may be summarised as follows:
1) The overall mortality rate after LMCA intervention with unrestricted use of DES appears to be in the range of 4% at one year, which drops to 2.3% when the cardiac mortality alone is considered. The observations that non-cardiac mortality may significantly contribute to total mortality is not an unexpected finding. Patients included in the current survey, in line with what is recommended by current guidelines, have major contraindications to surgery, often due to the presence of relevant comorbidities; they may play a role in explaining long-term survival in this patient population almost as much as LMCA disease does. This notion needs to be considered in planning future investigations comparing percutaneous versus surgical LMCA treatment, where patients with serious concomitant disease, in order to be eligible to both treatments, have necessarily to be excluded so that the overall and non-cardiac mortality might be correspondingly lower in such a setting.
2) The rate of sudden death in our series of patients was below 1%. Importantly, our data do not support the idea that SD may be causally connected to in-stent restenosis. One case of SD occurred 8 days after treatment, while the second case occurred 3 months after intervention, immediately after the discontinuation of antiplatelet therapy. The temporal relationship with intervention in the first case and awareness that anti-platelet treatment discontinuation is the biggest predictor of early and late stent thrombosis in the DES era7, argues in favour of stent thrombosis as the most likely mechanism of SD in our cohort of patients.
3) The rate of stent thrombosis appeared to be in the range of 1% in the worst possible scenario, where confirmed or suspected thrombotic events were pooled. This finding favourably compares to the rate of stent thrombosis in the BMS era and does not seem to differ from what was previously reported in consecutive series of patients undergoing liberal DES-supported intervention7,8.
4) A clear clustering of fatal and non-fatal hard adverse events in the first three months after treatment was noted, with a 50% decrease in death or MI form 3 to 6 months, which appeared to stabilise thereafter, at least until 12 months. This drop of major adverse events after three months is in full agreement with what was previously reported in the BMS era2. As previously discussed, this was interpreted as the hazard of developing in-stent restenosis after LMCA treatment. This speculation was supported by the notion that intimal hyperplasia after BMS is known to peak 14-16 weeks after intervention. The finding that after DES implantation the same clustering of adverse events persists in the first months after treatment, offers the unique possibility to challenge previous beliefs about the malignant role of intima hyperplasia in this cohort of patients. In the longest available angiographic follow-up after drug eluting stent implantation, neointimal growth has been shown to mildly non-significantly progress even beyond one year9. Recently, Wessely et al reported on two patients treated with sirolimus-eluting stent in the left anterior descending artery and right coronary artery who presented at 13 and 19 months, respectively, with recurrence of symptoms and angiographically confirmed in-stent restenosis. Of note, both patients had undergone previous coronary angiogram at 7 months, which showed no evidence of intima growth at that stage10. Finally, in 15 patients who underwent treatment with DES for LMCA disease at the Thoraxcenter, serial angiographic follow-up at 6 and 12 months after the index procedure was recently carried out. Of note, the late loss which was observed at 6 months [median (IQR): 0.29 (0.07-0.4) doubled at 1 year [0.63 (0.37-0.76), p<0.001], with two patients presenting with mild intima hyperplasia at 6 months who developed late in stent restenosis at 1 year (JACC, in press).
Thus, current evidence confirms the notion that intimal hyperplasia after DES implantation may be much more delayed with respect to what was observed in the BMS era. Of note, even after LMCA intervention with DES, the same clustering of adverse events in the first few months afterwards, as it has previously reported in BMS treated patients, seems to occur according to the present survey. Thus, when taken together, available data may argue in favour of mechanisms different from intimal hyperplasia as putative explanation for the different rate of death or MI over time in this patient population.
5) In those patients who refused to undergo angiographic follow-up (n=65), no increase of adverse events was noted in the current survey. Notably, no fatal or non- fatal major adverse event occurred in this group of patients. We cannot rule out the possibility that these findings may be subject to a considerable selection bias. Ideally, a randomised prospective study allocating consecutive LMCA treated patients to protocol-mandated angiographic surveillance versus conservative management would be in demand to evaluate the safety of avoiding systematic angiographic examination in this cohort of patients. However, the present study does not support the previous recommendation that routine angiographic surveillance seems to be mandatory in LMCA patients undergoing percutaneous treatment. The fact that early angiographic control may underestimate the real incidence of in-stent restenosis, coupled with the potential hazard to undergo multiple angiographic examinations over time, should not be neglected if current recommendations to obtain multiple early angiograms in this patient population is followed.
Conclusion
In conclusion, based on the largest series of patients undergoing unprotected and elective LMCA intervention ever reported, the current study shows that in this cohort of patients: i) the rate of SD, cardiac death and stent thrombosis favourably compares to what is reported in patients receiving treatment for non-LMCA lesions; ii) in this patient population at high surgical risk, the contribution of non-cardiac death to overall mortality may be substantial; iii) a clustering of adverse events in the first months after treatment, as was true in the BMS era, could be confirmed in patients receiving DES implantation. This finding, coupled with the rising notion that the peak of intima hyperplasia may be much delayed after DES with respect to BMS and that no safety concerns emerged in the current analysis in those patients refusing to undergo angiographic control, may suggest that postponing angiographic follow-up beyond the conventional 6 months does not affect the outcome of these patients, while this would allow us to monitor the vessel status when the intimal growth has most probably ceased and the net lumen gain is likely to be maintained over time.
There remains considerable uncertainty, however, around the need to perform or not perform a mandatory angiographic control in patients undergoing LMCA intervention. Thus, no clear recommendation can be made in this setting. We cannot exclude the possibility that in selected patient subsets “malignant” early intimal hyperplasia takes place which may contribute together, explaining the clustering of adverse events in the first few months after treatment.
In conclusion, our survey shows that in patients undergoing elective intervention with DES for unprotected LMCA stenosis, the short and mid-term outcome is favourable. The great majority of major observed cardiac events occurred in the first few months after treatment, while at the time, when intima hyperplasia is expected to peak (beyond 6 months), there was no detectable increase of death or myocardial infarction. Prospective, randomised, controlled investigations are required in order to evaluate the safety in avoiding systematic angiographic surveillance in this group of patients.

