Abstract
Aim: To report the periprocedural and long-term results of using the Amplatzer septal occluder for primary closure of post myocardial infarction ventricular septal defects.
Methods and results: Transcatheter closure was considered in patients with significant left-to-right shunting and defect anatomy and location thought to be suitable for closure with such a device. From December 1999 until February 2005 eleven patients (9 males) aged 52-81 years (mean 67,9) underwent an attempted closure. The time from the onset of infarction to the procedure ranged between 2 days and 58 weeks (mean 15,4 weeks). There were three patients in an acute phase of infarction (three weeks or less). They were in critical condition and required inotropic and ventilatory support. Eight patients (all in a chronic infarction phase) were hemodynamically stable and in NYHA class III-IV (6 patients) or class II (2 patients). A successful device implantation occurred in all but one patient, in whom a 26 mm occluder pulled through a 16 mm defect on day 8 of infarction. An infarct exclusion surgery was successfully performed in this patient. In the remaining 10 patients, the defect size ranged 8-21 mm (mean 14,3), and the devices 11-30 mm (mean 19,3) were implanted. The procedure and screening time ranged 134-286 (mean 187,2) and 23-90 minutes (mean 43,6) respectively. The successful implantation did not clinically succeed in both patients with the acute septal rupture - they died 2 and 15 days after the procedure. In the eight patients in whom the procedure was performed late (3,5-56 weeks) after the infarction onset, the defect was either completely closed or the shunt was insignificant, and they improved dramatically. In the most recent follow-up from 1 to 62 months (mean 25,5), the patients have been alive and feeling well, and in NYHA I or II class.
Conclusion: Primary transcatheter closure of postinfarction ventricular septal defects may be an alternative to surgery in patients with suitable anatomy and completed necrosis. In our experience, primary transcatheter closure of ventricular septal defects in patients who are in the acute phase of infarction does not improve their survival.
Introduction
The ventricular septal rupture is an uncommon complication of myocardial infarction, with a reported incidence of 0,2% in the thrombolytic era1. The outcome remains extremely poor, and 94% of patients treated medically die within 30 days1. Surgical defect closure still remains the only therapeutic option improving survival, and immediate operative intervention in patients with septal rupture is recommended regardless of their clinical status2.
There are single reports based on small series of patients, or case reports, on transcatheter closure of postinfarction ventricular septal defects (post-MI-VSD), and experience is limited3-9. The Amplatzer devices provide new potentials and options for transcatheter treatment of this complication. In this report we present our periprocedural and late results of primary closure in selected patients, of post-MI-VSDs using the Amplatzer septal occluder - a device originally made for transcatheter closure of secundum atrial septal defects10.
Patients and methods
Patients
Transcatheter closure was considered in patients with significant left-to-right shunting (pulmonary/systemic flow ≥1.5), defect anatomy and location thought to be suitable for closure with the device. The size and location of each defect was assessed with transthoracic, or transoesophageal echocardiography.
The first procedure was performed in December 1999 and, to the best of our knowledge, this was the first reported4 successful primary transcatheter closure of the post-MI-VSD with the Amplatzer occluder. Until February 2005 eleven patients (9 males) aged from 52 to 81 years (mean 67,9) underwent an attempted closure of post-MI-VSDs. Data of the ten patients, in whom the device implantation was successful, are presented in the table. None of the patients had primary surgical repair of the defect. The myocardial infarction preceding the septal rupture was anterior/apical in 6 patients, and inferior/posterior in 5 patients. The time from the infarction onset to the procedure ranged from 2 days to 58 weeks (mean 15,4 weeks). There were three patients in an acute phase of infarction (three weeks or less) and the remaining 8 had chronic defects. All three patients in the acute phase of infarction were in critical condition and required inotropic and ventilatory support. The remaining eight patients (all in the chronic phase) were hemodynamically stable and in class III-IV (5 patients) or II (3 patients) according to the New York Heart Association.
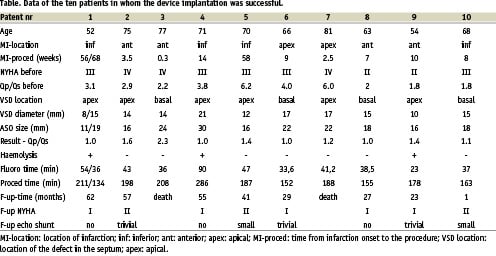
All the patients had a “simple” septal rupture11 with a direct through-and-through communication across the septum. The defect was localized in the apical segment in 7 patients, and in the basal segment of the septum in 4 patients. Pulmonary-to-systemic flow ratios ranged from 1,8 to 6,2 (mean 3,4).
Coronary angiography was performed prior to defect closure in all patients. Two patients underwent primary angioplasty. None of the patients required post-procedural coronary intervention.
Right and left heart catheterisation was repeated directly after the device implantation, or before discharge. Transthoracic echocardiograms with colour Doppler flow mapping were repeated at follow-up visits. The degree of residual shunt was assessed in echocardiography by means of measuring the width of the colour jet exiting through the septum12. The patients were routinely maintained on aspirin for at least 6 months following the procedure.
The informed consent regarding the experimental nature of the procedure was obtained from each patient.
Procedure
The Amplatzer septal occluder (AGA Medical Corp., Golden Valley, Minnesota, USA), originally designed for atrial septal defect closure, was used in all the patients. The device is a self-expandable circular double-disc frame made of windings of Nitinol wire, with a central connecting waist. The discs extend the waist by 5 to 7 mm. The size of the occluder is determined by the diameter of the waist, which is made to fit the defect. Waist diameters vary from 4 to 40 mm. The device stents the actual defect and the occlusion is achieved partly by the thin polyester patch inserts, but mainly by in situ thrombosis and subsequent endothelialisation10.
The implantation technique was similar to that described originally by Landzberg et al.3, and was presented in details in our previous report4. According to our atrial septal defect closure protocol, the first two procedures were done in general anaesthesia, with multiplane transoesophageal echocardiography guidance. Transthoracic echocardiography was used for the following procedures, and general anaesthesia only for clinical reasons. As a rule, we applied the femoral arterial and jugular venous approach with the arteriovenous guidewire loop creation.
A 6 French balloon-wedge pressure catheter (Arrow International Inc.) was used to cross the defect from the femoral arterial approach and manipulated into the right or left pulmonary artery or the right atrium. A 360 cm, 0.035 inch guidewire was then introduced through this catheter, its tip snared with a loop of the Guenther vena cava retriever (Wiliam COOK Europe, Denmark), and then extruded via the right internal jugular vein, thus providing an arterial-venous continuous access.
The defect balloon sizing was used only in the first patient. In the remaining ones, the device was chosen according to the defect size determined by echocardiography. Devices larger at least 2 mm than the maximal defect dimension were used. An Amplatzer® introduction system with a long sheath was passed from the jugular venous access through the defect into the left ventricle. Subsequent delivery proceeded similar to that for atrial septal defect closure10. The dilator and the long wire were removed. The device screwed onto the delivery cable was loaded and introduced through the sheath. The distal disc was extruded in the left ventricle and the entire system withdrawn to oppose the disc against the wall of the ventricular septum. The second disc was then deployed on the right side of the septum by withdrawing the sheath. After the placement, gentle pushing and pulling with the delivery cable proved the fixation of the device and its mechanical stability. Once echocardiographic studies confirmed proper position of the device, it was unscrewed by counterclockwise rotation of the delivery cable.
Results
Procedures
A successful device implantation occurred in all but one patient. An attempt to close an apical defect was made in a 70-year-old diabetic female on day 8 of infarction. A 13x16 mm defect was measured by echocardiography. A 20 mm occluder was implanted, but pulled through into the right ventricle before the detachment. A 26 mm occluder was then implanted but pulled through also. An infarct exclusion surgery was successfully performed in this patient on the same day. The surgeon’s impression was, that a 40 mm device would probably close the defect (figure 1). In one patient (table: patient nr 1) two defects were closed during the separate sessions. In another patient it was impossible to introduce the long delivery sheath over the guidewire across the defect. Most probably this was a result of the winding of the wire on the tricuspid chordae. During the second session, this time successful, a balloon wedge pressure catheter was used instead of a multipurpose catheter, to reach the pulmonary artery from the jugular vein, thus creating the arteriovenous guidewire loop.
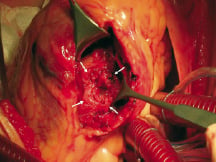
Fig. 1. Surgical view of the acute septal rupture from the left ventricular side: the defect with very irregular margins; difficult to see the actual interventricular communication (arrows); large necrotic area with friable tissue surrounding the hole.
The defect size ranged from 8 to 21 mm (mean 14,3), and the devices from 11 to 30 mm (mean 19,3) were implanted.
The procedure and screening time ranged 134-286 (mean 187,2) and 23-90 minutes (mean 43,6) respectively.
Periprocedural period
The successful device implantation did not clinically succeed in both patients with the acute phase of septal rupture. In a 77-year-old patient admitted in cardiogenic shock, the procedure was performed on day 2 of infarction (table: patient nr 3). A significant shunt persisted despite the proper placement of a large device in the septum. The residual leak was related to the additional defects in the region of the main hole. The patient died 2 days after the implantation due to the multiorgan failure. In a 81-year-old male with a large left ventricular impairment, there was no clinical improvement despite the successful immediate closure of the shunt on infarction day 15 (table: patient nr 7). He died 12 days later of multiorgan failure and gastro-intestinal bleeding.
In the remaining patients, in which the procedure was performed late (3,5-56 weeks) after the infarction onset, the defect was either completely closed or the residual shunt was insignificant.
At the periprocedural period, a transient self-limiting haemolysis was observed in three patients. One of them required blood transfusion. None of the remaining patients required periprocedural blood transfusion. A transient, complete atrio-ventricular block occurred during the arterio-venous guidewire loop creation in two patients. There was no device embolization.
Follow-up
The patients were followed for 1 to 62 months (mean 25,5). All eight patients were alive and feeling well. All of them improved dramatically. In the most recent follow-up, they were in NYHA I or II class. Transthoracic echocardiography demonstrated the defect to be closed in 3 patients, in 3 patients there was a trivial residual shunt, and in 2 patients a small one.
Discussion
For clinical practice, three separate groups of patients with post-MI-VSD must be recognized:
-patients with the acute septal rupture,
-patients with the chronic septal defect, and
-patients with the residual or recurrent defect following the initial surgical repair.
In the acute period the defect evolves. Within the first three to five days after the infarction, coagulation necrosis develops and lytic enzymes released by neutrophils cause the disintegration of necrotic myocardium. The site of the septal rupture becomes surrounded by the fragile necrotic tissue (figure 1). Due to the continued necrosis, resorption and retraction of the infarcted tissue, the defect enlarges in size11. The healing after the myocardial infarction was not remarkable until after the third week13. Within several weeks, the defect evolves into the chronic phase, the septum becomes fibrotic and the scar develops (figure 2)11.
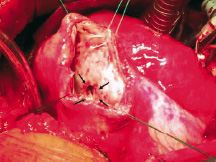
Fig. 2. Surgical view of the chronic post-infarction defect from the left ventricular side: round-shaped hole with very discrete margins (arrows); firm scar tissue surrounding the defect.
Although published in forms of case reports or small series, transcatheter closure has been applied in all the groups of patients.
Acute ventricular septal rupture
In all of our three patients with the acute septal rupture, the procedure was unsuccessful, or failed to improve their survival. This outcome represents three possibilities of the procedure failure in such patients: the failure to implant the device, the failure to close the shunt irrespective of proper device implantation and the “clinical failure” irrespective of the shunt closure in a dying patient.
Similar experience was reported by Landzberg et al., who failed to achieve improved survival in all four patients with acute ventricular septal rupture, in whom a primary closure with the double umbrella-type devices was performed. This was due to the enlargement of the previously covered ruptured segment3.
The Amplatzer occluders, including the muscular VSD and postinfarction VSD occluders used recently5,6,8, may have some advantages over the previously used devices for transcatheter closure of congenital or postinfarction ventricular septal defects3,14. In contrast to patch-like devices, the Amplatzer is a self-centering one and it stents the actual defect. The occlusion is achieved by the central part of the occluder, and is therefore less affected by septal wall irregularities. The self-expanding properties of the device are particularly useful in case of the defect enlargement.
In the US Registry of transcatheter closure of postinfarction ventricular septal defects using the new Amplatzer muscular VSD occluder, 18 patients were attempted and 8 of them had primary transcatheter closure. Eleven patients were alive and well, “two of them acute patients without prior attempts to close the VSD”. There were no data regarding the number of patients who had primary closure in the early stage of infarction6.
Our experience provides no data, that early closure of post-MI-VSDs can improve survival. It is possible that in selected patients with the suitable anatomy of the defect, the transcatheter closure with Amplatzer devices could be beneficial. In such cases much larger devices (even two times larger than the dimensions of the defect) should probably be used. We believe that, in general, early surgery with the infarct exclusion, septal stabilization, left ventricular remodelling and revascularization (if necessary), offering 60-70% perioperative survival2,15,16,17, is a better option for the severely ill patients with the acute septal rupture.
Chronic ventricular septal defect
In all of our eight survivors the procedure was performed beyond the third week of infarction, outside the acute phase. None of them had previously been operated. The device implantation was successful in all these patients and resulted in a dramatic clinical improvement lasting up to 5 years. Szkutnik et al. reported the primary closure of post-MI-VSD beyond the acute period in 4 patients in which three survived9. We believe that patients with heart failure and with the defects with completed necrosis and suitable location are the ideal candidates for the transcatheter closure with the Amplatzer device. It has been long believed and recommended, that surgery be delayed ideally for 3 to 6 weeks to allow the margins of the infarcted muscle to develop a firm scar18. Probably the same should apply now to transcatheter closure.
Residual or recurrent ventricular septal defect following initial surgical repair
Following initial surgical repair, a VSD recurs in up to 20% of the cases. This is due to the infarct extension leading to patch dehiscence or creation of a new VSD19. According to the single reports on 1 to 10 such patients the outcome of the transcatheter closure performed early or late after the initial surgical repair is generally good3,7. Although the complete closure is uncommon, the procedure is suggested an alternative treatment with lower morbidity than the repeated surgery. The likely explanation of the improved outcome of these patients is, that such patients generally present late after their initial myocardial infarction, and that the prior patch repair may provide a solid support for the device for at least a part of the circumference of the defect.
The technique used (retrograde crossing of the ventricular septal defect and creating the arterio-venous loop by snaring of a long guidewire from the jugular venous approach) appeared to be successful in all of our patients with different defect locations. The use of a balloon-tipped-catheter ensured crossing the septum through the major hole, thus avoiding minor additional fenestrations. Right ventricular trabeculae would make it difficult to cross the central channel of the defect from the venous approach. The right jugular venous approach enabled to create the way for a long delivery sheath with minimal number of bends and turns. According to our experience, while creating the guidewire loop, a balloon-tipped-catheter should also be inserted through the jugular vein, in order to avoid the collision with the tricuspid valve chordae.
With exception of the first patient, we did not perform balloon sizing of the defect. Echocardiographic measurements appeared to be sufficient in the patients with the chronic defects. The oversized devices (at least 2-4 mm larger than the defect maximal echo-measured dimension) should be used to obtain the firm and stable device position in the septum.
Due to the extremely fragile and irregular margins, the ongoing necrosis, resorption and retraction of the infracted tissue, the echocardiographic defect measurements are not reliable in the patients who are in the acute phase of infarction. It is questionable whether balloon sizing should be performed in these patients. This maneuver can potentially lead to the enlargement of the initial hole and should probably be avoided.
Haemolysis is a complication one has to be aware of in case of a high velocity jet, when a stiff foreign material is present. This may cause serious treatment obstacles if it does not resolve itself.
The procedure is suitable for the patients with a “simple”11 septal rupture, with a discrete defect and a direct through-and-through communication across the septum. The patients with a “complex” septal rupture with irregular, serpiginous tracts are not the candidates for transcatheter closure with this device. The defect has to be positioned far enough from the mitral and tricuspid valve apparatus (in the patients with the inferior infarction and the basal defects), in order to avoid the impinging of the device on the valves.
Summary
The primary transcatheter closure of post-MI-VSDs may be an alternative to surgery in the patients with suitable anatomy and completed necrosis, or the treatment of choice for the patients at high surgical risk. In our experience, primary transcatheter closure of ventricular septal defects in patients in the acute phase of infarction does not improve their survival.

