Abstract
Aims: To identify features predictive of hospital coronary artery bypass graft (CABG) surgery in patients with acute coronary syndromes (ACSs).
Methods and results: Data from 17,434 patients enrolled in an observational study were analysed. Patients in private hospitals were more likely to undergo CABG than those in public hospitals (10.3% vs. 6.9%, P<0.01); CABG was more frequent in the USA than in Europe (11.9 % vs 3.5%, P<0.01). Clinical features independently predictive of CABG on multivariable analysis included no previous CABG, male sex, history of angina, hypertension, hyperlipidaemia, or diabetes, no history of atrial fibrillation or congestive heart failure, ST depression in multiple territories, and absence of ST elevation. These factors were assigned a score to quantify the likelihood of CABG (c-statistic 0.69). This score was predictive regardless of ACS subgroup (c-statistic 0.65-0.71) and remained predictive across institutions regardless of the frequency with which CABG was performed. The score was of greatest clinical utility among hospitals performing CABG in >10% of their ACS patients.
Conclusions: Identifying ACS patients likely to undergo CABG using clinical features alone remains difficult. In hospitals with higher rates of surgical revascularisation, a subgroup of patients with an approximate 30% likelihood of CABG can be identified. Therapy in these patients can be tailored to minimise bleeding risk without compromising outcomes.
Introduction
Contemporary therapy for patients presenting with an acute coronary syndrome (ACS) includes antiplatelet and anticoagulant therapy.1 The benefit becomes apparent within hours of administration so that patients are optimally managed by the provision of these therapies in the emergency department on presentation. However, the modes of action of these drugs increase the likelihood of bleeding, particularly in the context of surgical procedures.2-7 These complications can be minimised if surgery is deferred; however, this delay is not only inconvenient but is potentially dangerous because the patient remains at risk of an adverse coronary event while waiting for revascularisation without the benefit of optimal therapy.8
One useful strategy would be to identify patients on presentation who have a significant likelihood of requiring coronary artery bypass graft (CABG) surgery. Medical therapy could be tailored for these patients to ensure their management is optimised and the risk of peri-CABG bleeding minimised. However, this task is difficult because, among patients with ACS, the clinical factors associated with in-hospital CABG are poorly defined. Furthermore, there are significant local and international variations in the frequency with which CABG is performed in patients with an ACS,9 which means that the usefulness of any clinical tool to identify these patients must be evaluated in the context of an individual hospital’s propensity to perform CABG.
The objectives of this analysis were to identify both institutional and clinical factors associated with an increased use of CABG among patients with ACS. The clinical features were used to derive a score to predict the likelihood of in-hospital CABG. This score was designed to be applicable internationally across the range of ACS diagnoses and, depending on an institution’s propensity to manage patients with early surgical revascularisation, could theoretically be used to guide initial medical therapies.
Methods
Patient sample
Full details of the Global Registry of Acute Coronary Events (GRACE) methods have been published.10,11 Patients entered in the study had to be at least 18 years old and alive at the time of hospital presentation, be admitted for ACS as a presumptive diagnosis (ie, have symptoms consistent with acute ischaemia) and have at least one of the following: electrocardiographic changes consistent with ACS, serial increases in serum biochemical markers of cardiac necrosis, and/or documentation of coronary artery disease. The qualifying ACS must not have been precipitated by significant non-cardiovascular comorbidity (eg, trauma or surgery). Where required, study investigators received approval from their local hospital ethics or institutional review board.
Data collection
The study aimed to enrol an unbiased population and sites were encouraged to recruit the first 10 to 20 consecutive eligible patients each month. Data were collected by trained coordinators using standardised case report forms. Demographic characteristics, medical history, presenting symptoms, duration of pre-hospital delay, biochemical and electrocardiographic findings, treatment practices, and a variety of hospital outcome data were collected. Standardised definitions of all patient-related variables, clinical diagnoses, and hospital complications and outcomes were used. All cases were assigned to one of the following categories: ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) or unstable angina. Patients were diagnosed with STEMI when they had new or presumed new ST-segment elevation >1 mm seen in any location, or new left bundle branch block on the index or qualifying electrocardiogram (ECG) with at least one positive cardiac biochemical marker of necrosis (CK-MB >2x upper limit of normal of the hospital’s normal range, or total CPK >2x upper limit of normal of the hospital’s normal range; positive troponin I or T). In cases of NSTEMI, at least one positive cardiac biochemical marker of necrosis without new ST-segment elevation seen on the index or qualifying ECG had to be present. Unstable angina was diagnosed when serum biochemical markers indicative of myocardial necrosis in each hospital’s laboratory were within the normal range. Patients originally admitted because of unstable angina but in whom myocardial infarction evolved during the hospital stay were classified as having a myocardial infarction.
A total of 48 hospitals with catheterisation and cardiac surgery facilities in 12 countries in North and South America, Europe, Australia and New Zealand contributed data to this analysis. Patients transferred into an enrolling GRACE hospital were excluded because they lacked baseline data. The population included 17,434 ACS patients enrolled between April 1999 and December 2003. Patients were grouped according to whether or not they underwent CABG during hospital admission.
Statistical methods
Differences in patient demographics, clinical characteristics, and hospital management and outcomes between patients undergoing CABG (CABG+) and those who did not (CABG–) were assessed using the χ2 test for categorical variables (expressed as frequencies and percentages) and the Wilcoxon rank-sum test for continuous variables (expressed as medians and interquartile range).
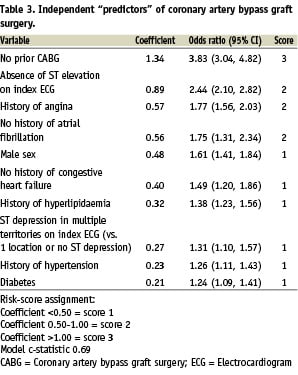
For the multivariable analysis, baseline and demographic factors with P<0.25 between CABG+ and CABG– groups were entered into a stepwise logistic regression (backward elimination). These included age; gender; medical history (angina, diabetes, renal disease, angiogram diagnostic for coronary artery disease, congestive heart failure, CABG, peripheral arterial disease, hypertension, hyperlipidaemia, atrial fibrillation); current smoker; initial creatinine; presenting Killip class; and ST-segment elevation, ST-segment depression in multiple territories (vs. single territory or no ST-segment depression), significant Q waves, T-wave inversions on index ECG. The final model included only factors significantly associated (P<0.01) with the use of CABG. A score was developed from the equation coefficients of the model (1 point for coefficient <0.50, 2 points for coefficient 0.50-1.00, 3 points for coefficient >1.00). The relation between score (continuous) and use of CABG was found to be linear by logistic regression. To evaluate the discriminatory ability of the score, we evaluated the area under the receiver-operating curve (c-statistic) for the regression model for CABG using the CABG score for the overall population and for selected subgroups of patients. SAS version 9.1 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Of the 17,434 patients with ACS, 1,334 (7.7%) underwent CABG. There were significant differences between these patients and those who did not undergo CABG (Table 1). Patients undergoing CABG were slightly younger and more likely to be male. Almost three-quarters of these patients had a history of angina prior to this presentation compared with 58% among those that did not undergo CABG. Patients who underwent CABG were more likely to be diabetic (30.0% vs. 25.5%, P = 0.0003), and to have a history of hypertension or hyperlipidaemia. They were less likely to be current smokers or to have a history of congestive heart failure, prior CABG surgery or atrial fibrillation.
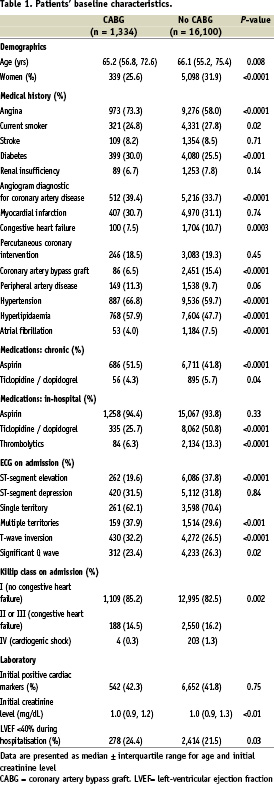
Patients presenting with ST elevation were under-represented in the group undergoing CABG (19.6% vs. 37.8%, P<0.0001), whereas the prevalence of elevated cardiac markers was equally represented among the two groups. Although presence of ST depression was comparable between the two groups, patients who underwent CABG were more likely to present with ST depression in multiple territories compared with patients who did not have CABG (37.9% vs. 29.6%, P = 0.0004). T-wave inversion was slightly more prevalent and Q waves slightly less frequent on the admission ECG among patients undergoing CABG. Patients undergoing CABG were less likely to have signs of heart failure on presentation although their estimated left ventricular ejection fraction was more likely to be under 40%.
Patients who underwent CABG were more likely to have been receiving aspirin at the time of presentation and slightly less likely to have been taking a thienopyridine, although the numbers receiving the latter as chronic therapy were small (4.3% vs. 5.7%, P = 0.04).
There were both institutional and regional differences in the frequency with which CABG was performed in patients presenting with an ACS. Patients presenting to private or non-teaching hospitals were more likely to have CABG surgery than those presenting to public or university hospitals. Patients in the USA were most likely to undergo CABG followed by patients in Argentina/Brazil, then Australia/New Zealand/Canada. Patients presenting to European sites in GRACE were the least likely to undergo CABG in hospital (Table 2).
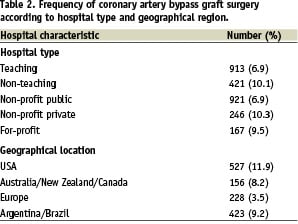
Differences in the frequency of CABG were noted among ACS subgroups. Patients with NSTEMI experienced the highest rates of CABG (9.9%), followed by patients with unstable angina (8.0%) and STEMI (5.5%) (data not shown).
Score for predicting CABG
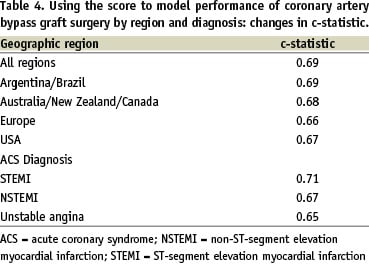
Factors that were independently predictive of CABG on multivariable analysis were identified and assigned a simple score to quantify the likelihood of CABG (Table 3). The c-statistic for predicting CABG use by the score was 0.69. This score was applied to patients within each geographic region, where despite the variation in frequency of CABG, the discriminatory ability of the score to predict CABG remained similar to the overall c-statistic (0.66-0.69). Similarly, despite the influence of diagnostic category of ACS on the frequency of hospital CABG, the score maintained similar discriminatory ability among STEMI, NSTEMI and unstable angina subgroups (c-statistic 0.65-0.71) (Table 4).
For the GRACE population as a whole, patients with the highest scores (from 13 to 15) had an approximate 18% observed frequency of CABG (data not shown). For the subset of patients selected for coronary angiography, those with scores between 13 and 15 had a 21% rate of hospital CABG (Figure 1), and the discriminatory capacity of the score was similar (c-statistic 0.68).
`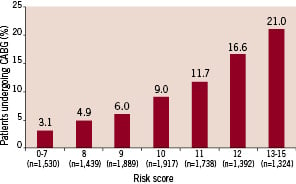
Figure 1. Use of coronary artery bypass graft surgery in patients undergoing coronary angiography. CABG = Coronary artery bypass graft surgery.
Hospitals were stratified into tertiles according to the relative frequency with which CABG was performed. The model remained consistently predictive (c-statistic 0.67-0.68) in hospitals with low, medium, and high frequencies of CABG (Table 5). Applying the score was of limited clinical value in hospitals performing CABG less often because the highest risk patients were relatively unlikely to undergo CABG: 9.4% of patients with a score >13 underwent CABG in hospitals with a low frequency of bypass surgery compared with 20.5% of patients in hospitals with a medium frequency. Among hospitals with the highest frequency of bypass surgery, however, >30% of patients with high scores underwent CABG during their admission (Figure 2).

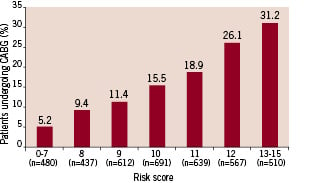
Figure 2. Use of coronary artery bypass graft surgery for patients undergoing coronary angiography at high-volume (CABG) hospitals (highest tertile). CABG = coronary artery bypass graft surgery.
Discussion
As patients with ACS derive increasing benefit from newer and more potent antithrombotic and antiplatelet therapies, the risk of excessive bleeding in patients undergoing CABG after receipt of these treatments has been increasingly recognised. Several studies have demonstrated a greater risk for postoperative haemorrhage among patients receiving low-molecular-weight heparin,6 abciximab,7 and especially clopidogrel.12 Although it has been argued that, in the ACS population, the benefit of early therapy justifies the risk of bleeding,13 this view is particularly unpopular among surgeons who have to cope with the consequences of these therapies. This issue is likely to be further highlighted with the uptake of more potent and longer lasting platelet and coagulation inhibitors.14-16
The benefits of bypass surgery are most robust in patients with extensive coronary disease, and the decision to operate is therefore determined on anatomical grounds.17 Our data illustrate that it remains challenging to identify, based on clinical characteristics, ACS patients who are likely to require surgical revascularisation before their anatomy has been defined. This analysis was performed in a contemporary international dataset that encompasses a broad range of hospitals and provides insights into variations in practice patterns of surgical revascularisation. We found a difference in the likelihood that patients would undergo surgery depending on geographical location as well as nature of the hospital; whether it was funded publicly or privately, or whether it was affiliated with a university. Despite this variation in practice, it was possible to develop a score that provided reliable prediction of the likelihood of hospital CABG in any ACS population. Once developed, the value of this predictive score was determined among patients with different ACS diagnoses in a range of facilities. The score remained consistent across all populations analysed despite a broad variation in the frequencies with which CABG was performed in individual institutions. This finding illustrates that, regardless of ACS diagnosis or practice patterns of any particular country, the same clinical factors are associated with a higher likelihood of surgical revascularisation. It would appear that the relative frequencies of the procedure depend on local practice patterns (for example relative frequency of coronary angiography, aggressiveness of interventional cardiologists vs. cardiac surgeons) rather than differences in patient characteristics.
Our data compare favourably with similar attempts by others. The Thrombolysis In Myocardial Infarction (TIMI) study group derived a score based on the “Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy thrombolysis in myocardial infarction” (TACTICS ) cohort, in which the variables that were independently associated with CABG were elevated troponin, prior stable angina, ST-segment deviation, male gender, history of peripheral vascular disease, and no prior CABG.18 The major difference from our own model is the predictive value of elevated troponin, suggesting that, although this was an important variable in this clinical trial population, in the real-world setting represented in GRACE, patients with elevated troponin are as likely to be managed either percutaneously or conservatively as by CABG. The TIMI group applied a score derived from these variables to a registry population and the associations remained significant. However, the registry population represented practice patterns from over a decade ago19; it remains to be seen whether this score remains applicable in a contemporary cohort with the more widespread application of percutaneous intervention.
A second, similar predictive score was developed from a single centre population of 688 patients undergoing coronary angiography.20 This score showed acceptable predictive value (receiver-operating characteristic curve 0.80±0.02) in a validation cohort from the same site. Importantly though, 36% of patients undergoing coronary angiography in this cohort were advised to have CABG. This is a greater rate of surgery than is seen in any of the 48 GRACE sites in our study, and suggests that the revascularisation practice may not reflect those adopted elsewhere. The present score has not been validated in other settings and its applicability to the general ACS population is yet to be determined.
One unique aspect of our analysis was the inclusion of patients with STEMI. Surgical revascularisation is less frequent in this group than in those with a less dramatic ACS presentation; however, it is often the sickest of these patients who require surgery, which is consequently associated with poor outcomes.21 Demonstration of a high CABG score in a patient with STEMI may be particularly important to optimise the selection of adjunctive pharmacotherapies and minimise the likelihood of further complicating a high-risk surgical procedure.
Although our model is predictive in all populations assessed, it is apparent that the clinical applicability varies depending on the frequency with which bypass surgery is performed in any institution. It is a simple matter for individual institutions, or individual cardiologists, to audit their own practice and determine the frequency with which their ACS patients undergo hospital CABG. Once this is known, it is possible to predict the likelihood that a patient will undergo CABG during the hospital admission. This in turn must be weighed against the risk and inconvenience of bleeding complications among patients undergoing CABG. In the clopidogrel in unstable angina to prevent recurrent events (CURE) study, major bleeding associated with CABG was reported in 9.6% of patients receiving clopidogrel within 5 days of CABG; an absolute increase of 3.3%.12 However, this may be an underestimation of outcomes outside the clinical trial setting. A review of the surgical literature reveals that the likelihood of re-exploration for early postoperative bleeding in patients on clopidogrel (an endpoint that was not tallied in CURE) ranges from 6 to 15% compared to rates of 1% among patients who have not been recently exposed to such therapy.2,3,5,22,23
In summary we have developed a score that allows the prediction of the likelihood with which patients with an ACS will proceed to CABG during their same admission. We suggest this score to be particularly applicable in sites where perioperative bleeding has proven to be a problem in patients following receipt of clopidogrel, or where surgeons are particularly robust in their refusal to operate on patients within 5 days of receipt of clopidogrel and the consequences of such delays place a burden on the patient and institution.
Acknowledgements
The authors would like to express their gratitude to the physicians and nurses participating in GRACE. Further information about the project, along with the complete list of participants, can be found at www.outcomes.org/grace. GRACE is funded by an unrestricted educational grant from sanofi-aventis, Paris, France, to the Center for Outcomes Research, University of Massachusetts, Worcester, MA, USA. We thank Dr Sophie Rushton-Smith who provided editorial assistance and was funded by sanofi-aventis.

