Restenosis prevention continues to be a challenge in interventional cardiology. Clinical trials on drug eluting stents (DES) suppressing neointimal proliferation by sustained release of antiproliferative drugs have shown excellent results in reducing restenosis. DES are well accepted in the prevention and therapy of coronary restenosis. Meanwhile, concerns have been raised that DES while being effective in reducing restenosis may be associated with an increased incidence of late thrombotic complications or death.
Restenosis caused by neointimal proliferation is a slow process, suggesting that prolonged local drug administration is necessary for effective inhibition. DES are characterised by a sustained drug delivery due to special features for slow release, mostly polymer matrixes. In this case, sustained drug release is essential because drug distribution from a DES to the arterial wall is inhomogeneous1. About 85% of the stented vessel wall area is not covered by the stent struts resulting in low tissue concentrations of the antiproliferative agent in these areas.
Cell culture experiments indicate that low drug concentrations require much longer exposition times to achieve sufficient inhibition of cell proliferation than higher concentrations2-5. Therefore, high drug concentrations on the stent struts including controlled and sustained release are mandatory for stent-based local drug delivery6 with the consequence of delayed and incomplete endothelialisation of the stent struts7. Furthermore, the polymeric matrixes on the stent embedding the antiproliferative drug could induce inflammation and thrombosis8,9. On the other hand, incomplete suppression of neointimal hyperplasia at the stent margins or between the struts may limit the efficacy of DES1,10.
Novel concepts to overcome the limitations of DES should avoid sustained drug release from stent struts including polymers or other sustained release technology. The drug-eluting PACCOCATH balloon (DEB) represents a novel option for the treatment of coronary and peripheral arteries. The DEB is a regular angioplasty balloon coated with paclitaxel at a dose of 3 µg/mm2 balloon surface. It requires no special handling. A key feature of the PACCOCATH is to facilitate the transfer of the antiproliferative agent from the balloon surface to the vessel wall during a short inflation time. The coating includes an X-ray contrast medium (iopromide) which improves the solubility of the drug and its transfer to the vessel wall.
The basic experiments leading to this device were performed by us based on the surprising discovery that sustained drug release as provided by DES is not a precondition for long lasting restenosis inhibition. The innovative coating technique enables for a controlled dose of paclitaxel to be released during dilatation as soon as the balloon is inflated inside the stenotic artery11. It allows for a homogeneous drug distribution to the arterial wall12.
Once exposed cells retain paclitaxel in vivo for six days even if plasma levels were far below the detection limit13. Preclinical studies have shown that brief contact between vascular smooth muscle cells and antiproliferative drugs can result in prolonged inhibition of neointimal proliferation4,5,11,14. It has been shown, that even after a short single dose application on human smooth muscle cell cultures, paclitaxel had a sustained anti-proliferative effect over 14 days without showing cytotoxic effects15.
Figure 1. Uncoated and paclitaxel-coated PTCA balloon catheters.
Initial drug concentration as achieved by the DEB is a substitute for sustained release. The drug administered only during the short inflation time of the balloon is subject to rapid dilution. As no additional drug is released from an implanted reservoir endothelial cells and their precursor cells migrating to the injured vessel segment16,17 are not exposed. Re-endothelialisation should not be inhibited because these cells entering the lesion from distant locations had no previous exposure to the drug and, therefore, maintain their capability to proliferate.
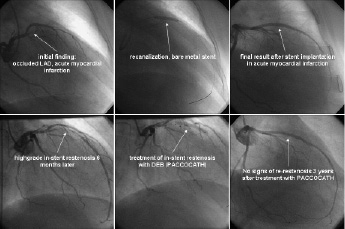
Figure 2. Patient from the PACCOCATH ISR I trial (Scheller 2006). Initial presentation with occluded LAD in acute myocardial infarction. Recanalisation by angioplasty and implantation of a bare metal stent. High grade restenosis six months later. Treatment of the in-stent restenosis with a 60 seconds inflation of the coronary PACCOCATH balloon. Angiographic long term follow-up of three years shows an excellent result without any signs of restenosis in the treated segment.
The PACCOCATH ISR I trial was a controlled randomised blinded first-in-man (FiM) study that investigated the use of paclitaxel-coated balloon catheters for treatment of coronary in-stent restenosis. Patients who were treated with the coated PACCOCATH balloon had significantly better angiographic results and concomitant 12-month clinical outcomes compared with patients treated with an uncoated balloon. The mean (±SD) in-segment luminal loss was reduced from 0.74±0.86 mm in the uncoated-balloon group to 0.03±0.48 mm in the coated-balloon group (p=0.002). There were no coating-related adverse events. Clopidogrel was given for only four weeks in both groups18. The results of this trial were confirmed by longer follow-up and the subsequent ISR II trial19.
Further evidence is available from treatment of peripheral arteries with the PACCOCATH balloon. The THUNDER trial (local Taxan with short time contact for reduction of restenosis in distal arteries) included patients with occlusion or stenosis of the superficial femoral or popliteal arteries. One hundred and fifty-four (154) patients were randomly assigned to treatment with uncoated standard balloon catheters and a regular non-ionic contrast agent, uncoated balloons and paclitaxel dissolved in the contrast medium, or with the PACCOCATH balloon for peripheral application and the regular contrast agent. The use of paclitaxel-coated angioplasty balloons during percutaneous treatment of femoropopliteal disease was associated with significant reductions in late lumen loss and target-lesion revascularisation21. Another German randomised multicentre trial in percutaneous treatment of femoropopliteal disease, the PACCOCATH FEM trial, showed similar results with the peripheral DEB.
A second generation coronary PACCOCATH balloon, the SeQuent™ Please (B.Braun Vascular Systems, Berlin, Germany) is filed for CE-mark. The aim of the ongoing PEPCAD clinical trial program (Paclitaxel-Eluting PTCA-Catheter in Coronary Artery Disease) using the DEB alone (SeQuent™ Please) or in combination with a thin strut CoCr stent (Coroflex™ DEBlue, B.Braun Vascular Systems, Berlin, Germany) is to study different indications with this novel device (Table 1).
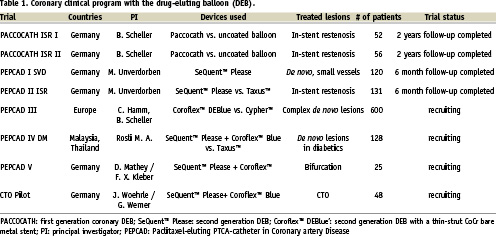
Meanwhile, the six months angiographic and clinical results of the PEPCAD I and II trials have been reported. PEPCAD I is a non-randomised, prospective multicentre study investigating the safety and efficacy of the paclitaxel-coated balloon in native small coronary vessels in 117 patients. Patients treated only with the DEB demonstrated a binary restenosis rate of less than 6%21. PEPCAD II is a randomised, prospective multicentre trial studying the safety and efficacy of the paclitaxel-coated balloon versus the Taxus stent in 131 patients with coronary in-stent restenosis. Compared to the Taxus stent, the DEB led to a significant reduction of angiographic late lumen loss and binary restenosis rate. Event free survival was improved with the DEB compared to the DES21.
The ongoing PEPCAD III trial is a 600 patient randomised European multicentre trial comparing the DEBlue stent system and the Cypher stent in complex de novo lesions. CE-mark for SeQuent™ Please is expected in summer 2008.
In perspective, the drug-coated balloon based on the PACCOCATH technology has the potential to improve the treatment of patients e.g., with coronary in-stent restenosis, lesions in bifurcations, small vessels, or other cases where stent implantation is not desirable or possible. With the drug-coated balloon there is no need for a stent. However, the combination with modern, flexible, thin, bare metal stents is another promising application. The treatment of peripheral arteries, where DES have shown limited efficacy, may become a future domain of the coated balloon.

