Introduction
Recent studies (see the overview of van Werkum and ten Berg in this current Eurointervention supplement) have linked an impaired response to antiplatelet therapy to a higher incidence of atherothrombotic events in patients undergoing percutaneous coronary intervention (PCI).1 As a consequence, several point-of-care tests to monitor the individual response to antiplatelet therapy are being used more frequently now in an effort to identify those patients who are at high risk for thrombotic complications (e.g. stent thrombosis). We address the question whether these tests are indeed reliable in identifying patients at risk, and therefore whether they should be used routinely in clinical practice.
The case
A 44-year-old man presented to our hospital with an acute infero-posterior myocardial infarction. He had no relevant past medical history and cardiovascular risk factors included a positive family history and smoking. Aspirin and unfractionated heparin were administered in the ambulance and clopidogrel (600 mg) immediately before the primary PCI. Coronary angiography confirmed a thrombotic occlusion of the right coronary artery which was successfully stented. The patient was included in a trial testing the predictive value of several platelet function assays to assess the patient’s response to clopidogrel therapy on day five. The results of these platelet function assays are depicted in Table 1. The patient experienced recurrent sudden onset chest pain on the eighth day after hospital admission. Repeat coronary angiography revealed a thrombotic occlusion of the stent, which was successfully treated with balloon angioplasty.
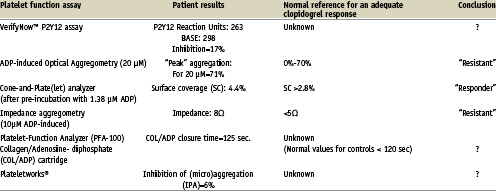
Platelet function assays
The question is: could the results of platelet function tests have alerted us and should we have changed the antiplatelet therapy? It is important to note that the results in our patient demonstrates that platelet function testing with several assays results in ambiguous and conflicting results. Some assays indicated that the patient’s platelets were insufficiently inhibited by clopidogrel, whereas other assays reported an adequate response. This concurs with the current literature, demonstrating that laboratory evaluation of the platelet response to a fixed “one size fits all” dosing regimen of aspirin and/or clopidogrel is highly dependent on which aspect of platelet function is being evaluated.2-4 Thus far, most of the studies linking platelet function with the occurrence of atherothrombotic events were performed with “classical” light transmittance aggregometry (LTA), a labour intensive method, and therefore not suitable to perform on a patient-to-patient basis in the catheterisation laboratory.5-10 The flow-cytometric VASP phosphorylation assay, which is more specific to the inhibition of clopidogrel’s target, the P2Y12-receptor, is also too labour intensive for routine use.11,12 The point-of-care platelet function assays (e.g. the VerifyNow™ system, the Platelet Function Analyzer [PFA-100] and the Plateletworks® assay), which are easy to use in the catheterisation laboratory, have been insufficiently validated and standardised to rely on at the present time.
Several steps have to be taken before individual dose-adjusting based on a reliable, simple and quick point-of-care platelet function assay can be successfully used in clinical practice:
1. The currently available platelet function tests need to be evaluated in large prospective trials that are based on clinical outcome.
2. The cut-off values for the assays, based on clinical outcome, need to be defined in order to separate non-responders from responders.
3. The evidence indicating that a change of therapy (e.g., increasing the dose of aspirin and, or clopidogrel, or switching to other antithrombotic therapies) will improve outcome without any safety concern such as bleeding has to be documented.
4. Individual monitoring of platelet function has to be cost-effective, especially in those subgroups where the risk of stent thrombosis is very low (e.g. patients with stable angina pectoris).
There is no solid ground for routine platelet function evaluation in patients who are scheduled for a PCI until these issues are adequately dealt with.
Do the objections stated above to identifying patients at risk for thrombotic complications preclude the use of these tests in clinical practice? The ACC/AHA/SCAI guidelines recommend that platelet function assays should be considered in those patients where a stent thrombosis may be lethal (e.g., unprotected left main or last remaining vessel). They also recommend that the dose of clopidogrel should be increased to 150 mg/ day when the usual 75 mg/day dose fails to obtain more than 50% inhibition of platelet aggregation (level of evidence 1C).13 Although there are no clinical data to support the use of higher doses based on platelet function assays, we concur with this statement, especially in high-risk patients (e.g. diabetes, obesity). Higher doses have been shown to be more effective in inhibiting platelet aggregation, while preliminary clinical data suggests that a higher dose does not increase the risk of bleeding.14
Finally, the answer to the question whether we could have been alerted by the results of platelet function assays performed in our patient is “no”. We await the results of the currently ongoing clinical trials before we can safely adjust the dose of antiplatelet therapy to an optimal level. The ongoing Aspirin Non-Responsiveness and Clopidogrel Endpoint Trial (ASCET) is trying to address whether aspirin resistance, defined using the PFA-100, can be linked to adverse cardiovascular outcomes in 1,000 coronary artery disease patients. The Gauging Responsiveness With A VerifyNow assay- Impact On Thrombosis And Safety (GRAVITAS) study will evaluate whether a change of therapy (double the dose of clopidogrel to 150 mg/daily in those who are below the required threshold of being a responder) results in an improved clinical outcome. The Do Point-of-care Platelet Function Assays Predict Clinical Outcomes in clopidogrel pre-treated patients undergoing elective PCI (POPular-study) aims to identify the most suitable point-of-care platelet function assay that predicts the occurrence of atherothrombotic events. And the STENT THROMBOSIS STUDY, which will include about 12,000 patients, will hopefully demonstrate the predictive value of the VerifyNow assays. If these trials indeed demonstrate that platelet function assays can be used to guide treatment-decisions and tailor-made antithrombotic treatment, several potential antiplatelet drugs (e.g. prasugrel) are eagerly waiting to be used in patients less responsive – or unresponsive – to clopidogrel.
Conclusion
Small studies have linked heightened on-treatment platelet reactivity to an increased risk for the development of stent thrombosis. Prospective trials of adequate size to confirm these findings are, however, lacking. Moreover, none of the currently available platelet function tests have been sufficiently validated and standardised to guide antiplatelet therapy for optimal effect. Therefore, until now, there is no case for a routine platelet function evaluation in patients who are scheduled for a PCI.

