Abstract
Background: Restenosis and a high incidence of new revascularisations reduce the long-term efficacy of percutaneous coronary intervention (PCI) in patients with multivessel coronary artery disease.
Aims: To determine the modality of utilisation and the clinical efficacy of drug eluting stents (DES) in a real world multivessel PCI scenario.
Methods: From July 2002 to December 2004, 1726 consecutive patients enrolled in the REAL Registry (Registro REgionale AngiopLastiche Emilia-Romagna) underwent elective multivessel PCI with multiple stents in at least two different vessels; among them, 939 (54%) received only bare-metal stents (BMS group), 288 (17%) only DES (DES group) and 499 (29%) were treated with BMS and DES in different vessels (MIX group). The incidence of major adverse cardiac events (MACE= death, myocardial infarction and target vessel revascularisation) during follow-up was assessed.
Results: Patients in the BMS group were older, diabetes was more frequent in the DES and MIX groups, while more lesions and more often a three vessel treatment was performed in the MIX group. In the DES group, lesions were longer, in smaller vessels and more often in the left main compared with BMS and MIX groups. In the MIX group too, lesions treated with DES were at higher risk for restenosis than those treated with BMS. Procedural success was similar in the three groups (98.9%). Notwithstanding the different risk profile, 12-month follow up did not show differences in clinical end points among the three groups. Multivariate analysis indicated that age, a modified Charlson’s comorbidity index and diabetes were independent predictors of death or AMI; total lesion length, use of only DES or MIX approach and treatment of left main were predictors of TVR, while left main treatment along with only DES use, modified Charlson’s index and reference vessel diameter independently affected the incidence of MACE. Use of at least one DES reduced the risk of TVR by 37% and MACE by 29%, while DES in every lesion treated reduced TVR risk by 37% and MACE by 39%.
Conclusions: In this large multicentre registry, DES were utilised in only half of the multivessel PCI procedures, mainly to treat high-risk patients and lesions. However, this selective use of DES was independently associated with a lower incidence of 1-year TVR and MACE. Whether increasing the rate of DES utilisation would further improve the clinical outcome remains to be investigated.
Introduction
In randomised studies on patients with multivessel coronary disease, coronary angioplasty and angioplasty plus stenting yielded similar results to coronary artery bypass graft (CABG) in terms of “hard” end points (death and myocardial infarction), while an excess of re-interventions (mostly percutaneous) at follow-up, namely target vessel revascularisation (TVR), was observed in all the percutaneous arms1-6. Recently, drug-eluting-stents (DES) have been associated with a dramatic reduction in the incidence of restenosis and the need for repeat interventions7-10.
The second arterial revascularisation therapies study, ARTS II11, although not randomised, did show excellent results of DES in multivessel PCI with an incidence of major adverse cardiac events (MACE) similar to that of the “historical” surgical arm of the ARTS I trial6. However a wide and unrestricted utilisation of DES to treat every lesion in every patient has two important limitations. Firstly, the costs of multivessel DES PCI are not yet covered everywhere by reimbursement, and the shortage of financing of the public health system seems to be an issue for the realisation of such a strategy in many countries12,13. Secondly there are no definitive data to demonstrate the equivalence or the superiority of multivessel DES PCI versus CABG in adequate cost/efficacy studies. In addition, to the best of our knowledge, no study demonstrating that treating all lesions with DES would be superior than a more selective approach has been published so far. These considerations, together with the knowledge that much of the restenosis risk largely depends on the specific patient and lesion characteristics lead to the possible development of an alternative strategy: DES utilisation for complex, high risk lesions and cheaper bare-metal-stent (BMS) for simpler and low risk lesions, even in the same patient. The clinical outcomes of this “mixed” approach, even if quite largely employed in the real world, has not been tested in any study, nor the results analysed in a large cohort of patients.
The purpose of this study was to investigate the modality of utilisation and the clinical impact of drug eluting stents in the percutaneous treatment of multivessel coronary artery disease in current clinical practice. We compared three different approaches: BMS only, DES only, and MIX (DES and BMS in the same patient) in a large prospective multicentre registry.
Materials and methods
REAL Registry
The characteristics of the REAL (Registro REgionale AngiopLastiche Emilia-Romagna) registry are described in detail in a previous paper14. Briefly, in July 2002, the date of the first availability of DES, the Regional Health Care Agency (RHCA) of the Italian Region of Emilia-Romagna (4 million residents) launched a prospective registry of PCI that collects data from all of the 13 interventional centres in the region. Clinical, anatomical and procedural data of all consecutive PCI procedures are entered in an electronic web-based database.
All patients gave written informed consent before the procedure.
General indications for DES implantation in patients with high-risk clinical or angiographic features were determined by a committee of interventional cardiologists and representatives of the RHCA at the launch of the Registry. However, these general recommendations did not represent mandatory guidelines, nor protocol-driven indications and the decision of implanting a DES in the single patient was left to the clinical judgement of the operator.
Patient population
The REAL Registry was screened from July 2002 to December 2004 and all the multivessel PCI procedures were identified and analysed in detail. Procedures had to be performed in patients resident in Emilia-Romagna and who received at least two stents in two different vessels and/or left main stenting. All patients with previous surgical or percutaneous coronary interventions were excluded from the analysis. Acute myocardial infarction (AMI) as the indication to PCI was excluded after a preliminary analysis showed AMI and shock (much more frequent in the BMS group) to be independent predictors of death and repeat AMI at follow up. The analysis was therefore limited to elective multivessel PCI with multiple stents. According to the type of stents received, patients were divided into three groups: BMS group, with implantation of multiple BMS in different lesions and vessels; DES group with implantation of multiple DES in different lesions and vessels; MIX group, with implantation of BMS and DES in different lesions and vessels in the same patient.
Procedure and post-intervention medication
The interventional strategy and device utilisation including DES were left to the discretion of the attending physician. Sirolimus eluting stents (SES) have been available since July 2002, while paclitaxel eluting stent (PES) have been available since March 2003. SES and PES were always available in all centres during all the study period.
Periprocedural glycoprotein IIb/IIIa inhibitors and antithrombotic medications were used according to operator’s decision and current guidelines. Lifelong aspirin was prescribed to all patients. One-month ticlopidine (250 bid) or clopidogrel (300 mg loading dose and 75 mg/d afterwards) was recommended for patients treated with bare stents, whereas the same treatment was extended to at least 3 months for patients treated with SES and to 6 months for patients treated with PES.
Definitions and follow up
Major adverse events (MACE) were defined as 1) death (cardiac and non-cardiac), 2) non-fatal myocardial infarction, or 3) target vessel revascularisation (TVR).
Myocardial infarction was diagnosed by a rise in the creatine kinase level more than twice the upper normal limit with an increased creatine kinase-MB. Cardiac specific enzymes were routinely assessed after the interventional procedure (at least two times up to 24 hours). Target vessel revascularisation was defined as repeat intervention (surgical or percutaneous) to treat a luminal stenosis located in the treated vessel inside or beyond the target lesion limit, while target lesion revascularisation (TLR) was defined as repeat intervention in the same coronary segment previously treated.
Lesion length and vessel reference diameter were visually estimated by the operators. On-line quantitative coronary analysis was allowed if required by the attending physician.
Administrative follow-up was obtained directly from the Emilia-Romagna regional health care agency through the analysis of the hospital discharge records and the municipal civil registries. All repeat interventions during follow-up were prospectively collected from the individual institutions as well and matched with the administrative data to adjust any eventual inconsistency. Hospital records were reviewed for additional information whenever deemed necessary.
Statistical analysis
Continuous variables were expressed as mean±SD and were compared using analysis of variance. Categorical variables were expressed as counts and percentages and chi-square test was used for comparison. All statistical tests were 2-tailed, and a p value <0.05 was considered as significant. The cumulative incidence of adverse events was estimated according to the Kaplan-Meier method and Cox proportional hazards models were used to assess risk reduction of adverse events. The variables included in the model were age, diabetes mellitus, previous myocardial infarction, Charlson’s comorbidity index15 modified excluding those variables already assessed independently (diabetes and previous myocardial infarction), left ventricle ejection fraction <35%, three-vessel intervention, proximal left anterior descending (LAD) and left main (LM) treatment, total lesion length, reference vessel diameter, ostial lesion, bifurcation, chronic total occlusion, only DES use and MIX approach.
A second multivariate analysis was performed including DES and MIX patients together in the model, in order to evaluate the use of at least one DES per patient versus no DES utilisation in multivessel PCI.
The association between pre-determined factors and adverse events is therefore expressed in terms of hazard ratio (HR) with 95% confidence intervals (CI), indicating a relative reduction in the rate of the events of interest when <1.
Results
Patient population
From July 2002 until December 2004, 16,499 PCI were included in the regional registry, of which 2,782 multivessel (22.7% of all PCI done in patients resident in the Emilia-Romagna region). Multivessel PCI with at least 2 stents in 2 different vessels were 2,374 (19.4%). The percentage of multivessel PCI increased during 3 years of the registry from 15% in 2002 to 24.2% in 2004.
Once all the patients treated for an AMI (n=321) and those with previous PCI or CABG (n=327) were excluded, 1,726 patients with multivessel PCI were evaluated, divided into 3 groups: 939 patients treated with multiple BMS (54%), 288 with DES only (17%) and 499 (29%) treated with BMS and DES in different lesions (MIX group). Overall, 46% of the patients with multivessel PCI received at least one DES; this percentage increased with time from 23.3% in 2002 (only DES 5.5% and MIX 17.8%) to 53.4% in 2004 (DES 23.1% and MIX 30.3%).
Only 203 patients (11.8%) had a staged PCI, while the vast majority had multivessel treatment in a single session.
Table 1 shows clinical and procedural characteristics of the patient population divided into the three groups BMS, DES and MIX. Patients treated with BMS only were older, while patients in DES and MIX groups more frequently had diabetes, three vessel disease and more complex angiographic and procedural characteristics. Procedural success was similar in all groups (98.9%). The anatomical characteristics of treated lesions are listed in Table 2 and show the preferential utilisation of DES in longer and more challenging stenosis.
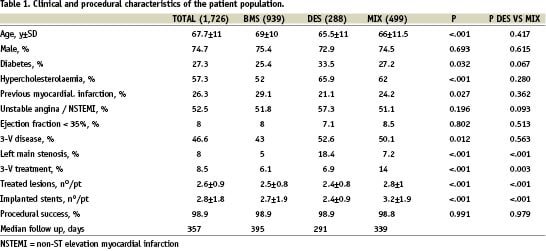
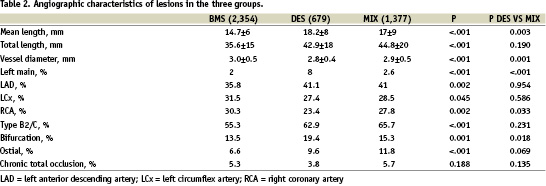
Within the MIX group, 648 lesions were treated with BMS and 654 with DES (plus 75 lesions with balloon angioplasty). Within this group, lesions treated with DES were longer (20.1±9.9 mm vs 14.6±6.9 mm, p<.001), and the reference vessel diameter was smaller (2.8±0.4 mm vs 3.0±0.5 mm, p<.001). Furthermore, the lesions treated with DES were more complex (B2/C type 76% vs 57%, p<.001), more frequently located in the LAD (64% vs 16%, p<.001), in bifurcations (21 vs 10%, p<.001) or ostial locations (15.0 vs 7.6%, p<.001) than those treated with BMS in the same patients.
Overall, 136 patients were treated for left main (LM) stenosis, 47 as a single lesion and 89 as part of a multilesion/multivessel PCI. The distribution in the three groups was as follows: 47 patients (34.5%) received only BMS, 53 (39.0%) only DES and 36 (26.5%) were in the MIX group. Sixty out of 136 LM stenosis were treated with BMS (mean lesion length 11.6±4.5 mm, vessel diameter 3.9±0.6 mm) and 76 with DES (mean lesion length 13.8±5.5 mm, p=0.014 vs BMS, vessel diameter 3.3±0.4 mm, p<0.001 vs BMS).
Median follow-up duration of the entire population was 357 days (range 90-1,003 days).
Clinical events
Figure 1 shows the 12 month clinical events. There were no significant differences among the three groups as far as incidence of AMI, death, TVR and cumulative MACE are concerned. TLR rates resulted similar in all groups (BMS 10.0%, DES 7.9%, MIX 7.9%, p=0.476). Notably, in the MIX group 60% of TVR were actually performed in a vessel treated with BMS.
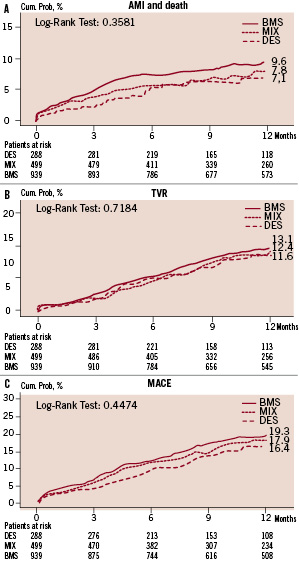
Figure 1. 12-month adverse events in multivessel PCI patients treated with only BMS, only DES or MIX approach. A: Cumulative risk of death or myocardial infarction; B: Target vessel revascularisation; C: Cumulative MACE (death, myocardial infarction or target vessel revascularisation).
Twelve month MACE incidence during the three years of the study did not change (19.6%, 17.6%, 19.1%, p=0.441 in 2002, 2003 and 2004 respectively) irrespective of the growing utilisation of DES. Routine angiographic follow-up was more frequent in DES treated patients. At 12 months, the incidence of non-clinically driven coronary angiography and/or TVR was 3.2% in BMS, 13.2% in MIX and 15.9% in DES group (p<.001).
Non-clinically driven 12 month TVR was 14.5%, 43% and 42% of total TVR in the three groups BMS, MIX and DES respectively.
Patients treated for LM disease showed significantly more events at follow-up (death and AMI, TVR and cumulative MACE) than patients without LM (Figure 2). In this group, patients who received only DES had less events than those treated with BMS and MIX (death and AMI 2.1% DES vs 20.2% MIX vs 25.5% BMS, p<0.001; TVR 7.9% DES vs 32.9% MIX vs 20% BMS, p=0.031; cumulative MACE 10% DES vs 54.2% MIX and 39.7% BMS, p<0.001).
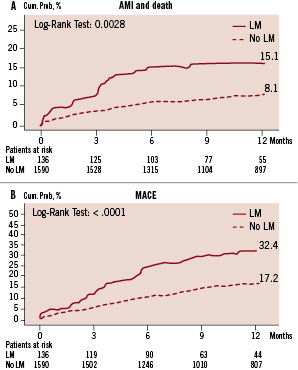
Figure 2. 12-month events in patients with and without left main disease. A: Death or non fatal myocardial infarction; B: Cumulative MACE (death, myocardial infarction or target vessel revascularisation).
In the entire population multivariate analysis with Cox regression model showed age (HR 1.024, CI 1.005-1.042, p=0.011), modified Charlson’s co-morbidity index (HR 1.310, CI 1.190-1.443, p<0.001) and diabetes (HR 1.496, CI 1.038-2.158, p=0.031) to be independent predictors of AMI and death, while LM treatment (HR 1.633, CI 0.968-2.755, p=0.066) and left ventricle ejection fraction <35% (HR 1.591, CI 0.959-2.641, p=0.072) showed only a trend toward increased risk of death and AMI without statistical significance.
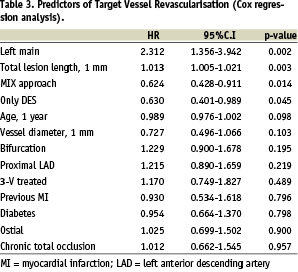
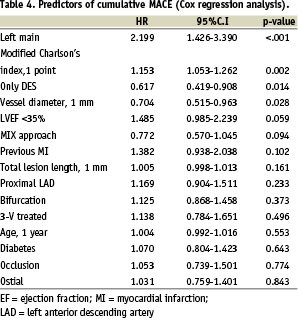
Tables 3 and 4 show predictors of TVR and cumulative MACE. Total lesion length, pure DES and MIX approach, left main treatment were significant predictors of TVR, whilst left main treatment, modified Charlson’s comorbidity index, pure DES utilisation and reference vessel diameter were the significant predictors of MACE.
When the factor “utilisation of at least one DES per patient” was introduced in the model combining the DES and MIX groups, this resulted significantly correlated with a 37% reduction of risk of TVR (HR 0.627, CI 0.450-0.873, p=0.006) and a 29% reduction of cumulative MACE (HR 0.714, CI 0.544-0.936, p=0.015).
Discussion
The first interesting finding of this study are the remarkably good results of elective multivessel PCI in a real world scenario; 12-month MACE rates ranged from 16.4% in the DES to 17.9% in the MIX and to 19.3% in the BMS group, notwithstanding the high-risk profile of the patients treated.
In the REAL registry, the lesions’ and the patients’ characteristics of the three groups and of DES-treated vessels in the MIX group, were consistent with a selective use of DES. In fact, DES were generally chosen to treat more complex lesions in patients at higher risk and this not surprisingly met the recommendations of the joint cardiological/cardio-surgical regional committee14, even if not responding to a predefined per-protocol driven use of BMS and DES. This strategy resulted in similar outcome in the three groups or, in other words, a selective use of DES reduced the risk of patients who received at least one DES at a level similar to less complex patients treated with BMS. However, these data indicate also a relative underutilisation of DES in clinical practice (only 46% of patients, with an increasing incidence during the three years reported in the registry). This fact, together with the reported 37% reduction of TVR and 39% reduction of MACE in patients treated solely with DES, might indicate that a larger utilisation of DES in multivessel PCI could lead to better results. There may be an underestimation of DES benefit on TVR and MACE due to the excess of routine coronary angiography and non-clinically driven TVR in patients treated with DES (MIX and DES groups), deriving from the early experience and diffusion of these devices and their utilisation in multivessel patients.
Patients’ clinical and procedural characteristics in the MIX group were highly complex, with a greater number of lesions treated and stents implanted. However, the good clinical results of multivessel PCI in this group with a limited TVR rate, even in BMS-treated vessels, reveals a shrewd therapeutic strategy that led to the utilisation of DES mainly in lesions at higher risk due to location and anatomical characteristics. These results, together with the improvement of BMS performance obtained with the most recently developed stents (CoCr alloy)16,17, as well as the suggestion that DES might be cost-effective only in specific high-risk subgroups17, indicates the need for a very precise evaluation of cost/effectiveness of the two different strategies, i.e. a mixed approach or an unrestricted DES utilisation in multivessel PCI. The relatively high cost of these devices is still the most important limiting factor in the applicability of an unrestricted utilisation of DES, especially in a limited resources scenario.
Percutaneous treatment of the unprotected LM coronary artery, was still associated with a high incidence of events (32.4% at 12 months). DES only treatment in this setting demonstrated a highly significant reduction of all events, with 10% MACEs rate at 12 months, which is intermediate compared with that reported by other DES studies18,19. However, the results of this subset analysis must be considered with caution, due to the small number of patients in the three groups, the significant differences among the three groups and the absence of a multivariate analysis in this subgroup of patients.
Another very interesting finding of the REAL registry is the lack of prognostic impact of diabetes on TVR (HR 0.954, 95% CI 0.664-1.370), although at the multivariate analysis diabetes was still associated with an increased risk of death and AMI (HR 1.496, 95% CI 1.038-2.158, p=0.031). This finding is in contrast with the results of another “real world” sirolimus-eluting stent registry, the RESEARCH Registry20, in which, anyhow, unrestricted DES implantation benefited, in terms of clinically driven TVR, diabetic patients less than non diabetics. As pointed out by Kastrati et al21 for restenosis after DES, other anatomical and procedural factors may be more important in predicting restenosis and TVR.
Limitations of the study
This study suffers the inherent limitations of all non-randomised studies. Patient population in the three groups was different and revascularisation strategy selection was at the discretion of the individual operators, although this bias has been partially obviated by multivariate analyses. On the other hand, this limitation may represent as well an element of strength of the study, which depicts a real world scenario with limited access to DES, and suggests a possible effective alternative to an unrestricted DES utilisation for anyone and for any lesion.
Prospective data collection and the complete administrative follow-up confer further solidity to our results.
Since the experience with DES began simultaneously with the institution of the registry, there was an excess of control routine coronary angiography 6 to 9 months after PCI in DES treated patients, and this has probably affected the TVR rate in DES and MIX groups.
Conclusions
In the REAL registry, drug-eluting stents were utilised in about one-half of multivessel PCIs, mainly to treat high-risk patients and lesions. This selective use of DES was associated with similar clinical results in the three groups (BMS, DES, MIX) characterised by different risk profile, and a clear risk reduction of 1 year revascularisations and adverse clinical events. Thus, a more widespread use of DES might further improve the clinical outcome. However, our experience also suggests that in multivessel PCI a study of selective use of BMS in lesions with pre-specified low-risk criteria might be justified on a cost-efficacy basis.
Appendix
The Emilia-Romagna REAL (Registro rEgionale AngiopLastiche) Investigators:
Regional Commission for Cardiology and Cardiac Surgery:
Umberto Guiducci (Chairman), Antonio Marzocchi, Aleardo Maresta, Paolo Alboni, Bruno Biagi, Roberto di Bartolomeo, Tiziano Gherli, Angelo Branzi, Anna Zucchini, Roberto Grilli.
Study Coordination and statistical analyses:
Roberto Grilli, Paolo Guastaroba, Elena Berti (Agenzia Sanitaria Regionale Emilia-Romagna, Bologna).
Clinicians from the following centres contributed to data collection:
Istituto di Cardiologia, Università di Bologna, Policlinico S.Orsola-Mapighi: Antonio Marzocchi, Francesco Saia, Cinzia Marrozzini, Paolo Ortolani, Tullio Palmerini; Unità Operativa di Cardiologia-Centro Interventistico, Ospedale S.Maria delle Croci, Ravenna: Aleardo Maresta, Elisabetta Varani, Marco Balducelli, Giuseppe Vecchi; Unità Operativa di Cardiologia Interventistica, Ospedale S. Maria Nuova, Reggio Emilia: Antonio Manari, Paola Giacometti, Stefano Fioroni, Vincenzo Guiducci; Divisione di Cardiologia, Ospedale Maggiore, Parma: Enrico Aurier, Luigi Vignali, Luigi Favaro, Alberto Menozzi; Divisione di Cardiologia, Ospedale Maggiore, Bologna: Pietro Sangiorgio, Gianni Casella, Andrea Rubboli, Giampiero Nobile; Unità Operativa di Cardiologia, Ospedale degli Infermi, Rimini: Giancarlo Piovaccari, Andrea Santarelli, Domenico Santoro, Nicoletta Franco; Unità di Cardio-Angiologia Interventistica, Casa di Cura Villa Maria Cecilia Hospital, Cotignola (RA): Alberto Cremonesi, Fausto Castriota, Enrico Ricci, Raffaella Manetti, Armando Liso, Kareem Oshoala; Laboratorio di Emodinamica, Ospedale di Ferrara: Gianfranco Percoco, Fabrizio Ferrari, Dario Barbieri, Monica Naldi; Laboratorio di Emodinamica, Policlinico di Modena: Giuseppe Geraci, Rosario Rossi, Fabio Sgura; Nuovo Ospedale S. Agostino, Modena: Stefano Tondi, Paolo Magnavacchi, Domenico Tosoni; Laboratorio di Emodinamica, Ospedale Morgagni di Forlì: Fabio Tarantino, Franco Rusticali, Marcello Galvani; Laboratorio di Emodinamica, Hesperia Hospital, Modena: Alberto Benassi, Giuseppe D’Anniballe, Luigi Steffanon; Ospedale di Piacenza: Alessandro Capucci, Francesco Passerini, Gabriella Giovannini, Maria Alberta Cattabiani.

