Introduction
Today’s standard practice of care for heart valve replacement requires open-heart surgery. Nevertheless current population with aortic valve disease is both older and has a higher incidence of associated cardiovascular or non-cardiovascular disease increasing the risk of conventionnal surgery.
Percutaneous endovascular therapies widely used in the treatment of coronary artery disease is still at a very early stage in the treatment of valve disease with few patients having recently experienced percutaneous or mini-invasive aortic valve replacement1,3.
Corevalve has developed a system called “CoreValve ReValving™ System” (CRS). This system is intended for Percutaneous Aortic Valve Replacement (PAVR) on a beating heart, avoiding open-heart surgery in patients presenting high risk or recused for surgical valve replacement. The present report describes a last generation device, pre-clinical data, anatomic requirements and technique of implantation of the CoreValve Aortic Valve Prosthesis.
Device description
The first generation of the ReValving™ System developed by CoreValve SA consisted of a commercialized bioprosthetic valve made of bovine pericardial tissue mounted and sutured in a self expanding nitinol stent. The prosthetic valve was delivered using a 25 French guiding catheter. It has been studied in 2 feasibility studies conducted in India-Venezuela and in Germany, respectively.
Technology is evolving and a major design change has been made by Corevalve with the replacement of the bovine pericardial tissue by a porcine pericardial tissue. The use of porcine pericardium improved the CRS allowing the use of a thinner delivery system (21 Fr instead of 25 Fr previously).
The CRS generation 2 consists of the following product elements:
– Percutaneous Aortic Valve Prosthesis (PAV) composed of a porcine pericardium tissue valve mounted in a tri-level self-expanding frame, designed for the replacement of the native aortic heart valve; the tissue valve is attached to the self-expanding frame in a manner similar to the attachment of tissue valves on their frame.
– Intra-vascular Delivery Catheter System (DCS), designed to house the PAV in the collapsed position for percutaneous delivery
– Compression and Loading System (CLS) designed to insert the PAV into the DCS in the hemodynamic room at the time of implantation.
All components of the CRS are single use.
CoreValve Percutaneous Aortic Valve Prosthesis (PAV)
The PAV (Figure 1a) consists of a self-expanding frame hosting a tissue valve.

Figure 1a. CoreValve Percutaneous Aortic Valve Prosthesis (PAV): views of the porcine pericardium valve sutured on the self-expandable stent’s most ultimate design.
Once deployed, the PAV is not retrievable from the site of expansion.
– The tissue valve is made of a single layer of porcine pericardium, manufactured by CoreValve, Inc. (Irvine, California, USA). The pericardium is built in a tri-leaflet configuration, attached to a scalloped skirt (which is also made of a single layer of porcine pericardium) on the inflow aspect of the valve using PTFE sutures (PAV sub assembly). Then the PAV sub-assembly is sutured to the self-expanding frame with 5.0 PTFE sutures. The shape of the skirt on the inflow side conforms to shape of the struts on the frame.
– The self-expanding frame (Figure 1b) is of a simple diamond cell design with different strut lengths and widths to accommodate expansion to a non-uniform cylindrical shape.
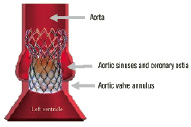
Figure 1b. The different parts of the self-expanding stent in regard to the human anatomy.
The overall stent lenght is 55 mm.
Its lower part has high radial force to push aside the calcified leaflets of the native valve, reduce the risk of para-valvular leaks and fix the valved stent at the level of the annulus.
Its middle part is shaped to host the tissue valve and to avoid interfering with the coronary ostia.
Its upper part increases quality of fixation and axial positioning of the valve in the ascending aorta.
The self-expanding frame is manufactured of Nitinol, a titanium and nickel alloy, having self expanding properties and well established biocompatibility. The self-expanding frame is at all times clearly visible under fluoroscopy.
Delivery Catheter System (DCS)
The DCS comprises an “over-the-wire” delivery catheter (Figure 2) which is designed around a central tube allowing it to slide over a guidewire up to 0,038 inch in diameter.
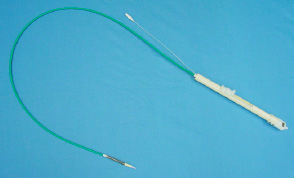
Figure 2. Delivery Catheter System (DCS).
The distal part of the catheter has a specially designed tip allowing for atraumatic insertion in the vessel. Covering this assembly is a protective sheath holding the prosthesis in a collapsed position. The end of the catheter shaft incorporates a handle which serves to execute the deployment of the prosthesis when positioned properly in the patient’s aortic annulus. The DCS is 21 French at the deployment end and 14 French at the shaft part.
The catheter’s handle features macro and micro adjustments to facilitate precise prosthesis deployment.
The DCS includes a radio-opaque marker located on the catheter tip to monitor positioning during implantation. Additional visualization guidance is provided by the frame profile itself, which is at all times clearly visible under fluoroscopy.
Compression and loading system (CLS)
The CLS 3rd generation (Figure 3, note that the presentation of this image is limited due to proprietary rights) is designed to radially compress the PAV to an optimal diameter to facilitate its loading on to the DCS.

Figure 3. Compression and loading system (CLS). The presentation of this image is limited due to proprietary rights.
This is done in the cath lab, few minutes before implantation.
Pre-clinical data
The CRS has been extensively tested in a long and complex process that included macroscopic, histological and ultrastructural (SEM) analysis of non-implanted PAV following DCS insertion, microscopic evaluation of the frame of the PAV after compression, fatigue analysis of the frame of the PAV, wear Testing of PAV, biocompatibility tests. They included as well human anatomy studies to determine the feasibility of the endovascular implantation of an aortic valve in humans, and animal feasibility studies done from October 2002 to February 2004 (4). The positive results of those tests led to the first human implantation of CRS 1st Generation in India in July 2004.
Anatomic requirements and technique of implantation
Anatomic requirements
Currently, the PAV exists two different sizes/model in regard to the ascending aortic diameter to be treated (<35 mm or <45 mm) and only in one size (26 mm) for aortic annulus diameter of < 23 mm.
Therefore, anatomic requirements with CRS Generation 2 included an aortic valve annulus diameter of < 23 mm and ascending aorta diameter of less than 45 mm, measured by transthoracic echographia or MRI.
For the access sides, common femoral arteries with diameter of < 7 mm avoiding very tortuous or heavily calcified iliac arteries that may make impossible insertion and endovascular access to the aortic valve.
Due to stent length, alignment of the valve in the proximal portion of the ascending aorta is also to be considered. An angle of 90° is ideal and facilitates valve-stent implantation. An angle of less than 60° should be avoided at this stage of the development.
Technique of implantation
The procedure is performed using general anesthesia.
Via humeral or radial artery access, a “pig tail” catheter is placed in the ascending aorta and an aortography performed. The optimal radiographic incidence that aligns in the same image the 3 aortic cusps to ease CRS implantation (generally 30° RAO - 20° Caudal) is selected.
A surgical cut down of femoral artery and vein for femoro-femoral temporary extracorporal circulation on one site and femoral artery for DCS insertion at the other is performed.
After crossing the aortic valve and placement of an extra support guide wire, the femoro-femoral assistance is activated. A valvuloplasty using a balloon angioplasty catheter of 23 mm in diameter is eventually performed.
A CRS according to the aortic and valve annulus diameters is selected, carefully rinsed and loaded into the 21 F delivery catheter.
Insertion through the access site and advance of the delivery system over the guidewire to the target aortic portion is performed under direct fluoroscopic visualization.
The delivery system is positioned so that the first cells of the stent are immediately (5 mm) after the level of the valve annulus. Correct CRS placement is assessed by angiography. When correct positioning is obtained, under fluoroscopic guidance the stent release process starts by slowly retracting the sheath actuator.
The position of the partially deployed prosthesis to ascertain if correct alignment with the annulus plane is regularly checked, using contrast medium injected through the diagnostic catheter if desired.
Slight proximal repositioning of a partially deployed prosthesis (<10% of the prosthesis) can be achieved by carefully and slowly pulling the entire delivery system in the vessel. Distal repositioning is not recommended under any circumstances. Then, continue retraction of the sheath is done until the entire length of the frame is deployed.
After the device has been completely deployed, the delivery system is retracted under fluoroscopic guidance then removed from the patient.
The femoro-femoral assistance is desactivated and both femoral access surgically sutured.
Routine post-procedural angiography is performed to verify results. A selective coronary angiography may be performed to control coronary ostia patency.
These generation 2 devices have been studied in a feasibility study conducted in Germany. See also the special case report from de Jaegere et al. published in this issue relating to the first treatment in the Netherlands using the CoreValve ReValving™ System.
Conclusion
The use of the generation 2 device has significantly improved ease and safety of Corevalve aortic valve prosthesis implantation compared to generation 1.
Currently, a European prospective multicentre, single arm safety and performance study is on-going. Eligible subjects will have severe symptomatic aortic valve disease with the addition of risk stratification (logistic EuroSCORE) reflecting a high peri-operative risk for the patient.

