Abstract
Percutaneous closure of patent foramen ovale (PFO) has been proposed as the treatment of choice for young high-risk patients who suffered cryptogenic stroke and/or peripheral paradoxical embolism. We sought to compare prospectively two different devices used for percutaneous PFO closure.
Prospective data were collected on 40 high risk patients (females: 38%, mean age : 44 ± 11 years, interatrial septal aneurysm >10 mm: 68%) who underwent percutaneous PFO closure after cryptogenic stroke (n = 38) or peripheral paradoxical embolism (n = 2). Chronologically, 20 patients were first treated by a PFO-Star (Cardia, Burnsville, MI) device. Then, 20 other patients received a Starflex occluder (NMT, Boston, MA). The primary endpoint was complete PFO closure at 6 months as assessed by transthoracic contrast echocardiography. Secondary endpoints were major peri- or post procedural complications and clinical recurrence at 1 year follow-up.
Baseline clinical and anatomical characteristics were comparable for both groups. Complete PFO closure was observed in 50% (PFO-Star) and 90% (Starflex) of patients (p=0.001) respectively. Major peri-procedural complications occurred in the PFO-star group only: right-sided device thrombus (1 patient) and aorto-right atrial fistula (1 patient). At 1 year follow-up, no clinical recurrence occurred.
In conclusion, despite the absence of clinical recurrence in this high-risk population with presumed paradoxical embolism, complete PFO closure at 6 months follow-up was significantly related to the type of closure device used.
Introduction
During fetal life, the foramen ovale allows oxygenated blood to shunt the pulmonary circulation by flowing directly from the right atrium to the systemic circulation. At birth the reversal of right-to-left pressure gradients induces a closure of the foramen ovale which should be definitive in the first 2 years of life. Nevertheless, autopsy studies reveal a patent foramen ovale (PFO) in about 27% of the adult population1. This entity has been reported in association with numerous clinical syndromes, including decompression sickness, migraine, platypnea-orthodeoxia syndrome and cryptogenic stroke2-4. The latter refers to stroke without definite cause despite an extensive neurological work-up. In patients younger than 55 years suffering a cryptogenic stroke, the prevalence of PFO varies between 40 and 70%5,6. The hypothesis that paradoxical embolism is the mechanism involved in cryptogenic stroke has been reinforced by several observations7-9. Recently, pelvic MRI venograms performed within 72 hours of onset of symptoms in young stroke patients suggested a 5 fold higher prevalence of pelvic deep vein thrombosis in patients with PFO compared to control10. Moreover, specific anatomical features have been identified as risk factors for stroke recurrence. Over a 4 years follow-up period, Mas et al. demonstrated a recurrence of 15.1% in young patients who had suffered cryptogenic stroke if their PFO was associated with a septum primum mobility above 10mm (atrial septum aneurysm: ASA)11.
Since Bridges first description of percutaneous PFO closure in 1991, the technique is now being considered as a treatment option for patients with presumed paradoxical embolism12,13. The literature reveals the use of different devices associated with a closure success ranging between 71% and 97%, and stroke recurrences between 0% and 22%14-16. Therefore, we prospectively compared the performance of 2 closure devices in a high risk population defined as patients with at least 2 cryptogenic stroke events and/or a PFO-ASA association.
Materials and methods
Patients: Patients were eligible for percutaneous PFO closure if they were considered at high risk for stroke recurrence. Therefore, inclusion criteria were: 1. the PFO-ASA association in the presence of at least 1 cryptogenic stroke and 2. cryptogenic stroke recurrence in the presence of PFO alone. All patients were referred after an extensive neurological work-up concluding to a cryptogenic stroke or transient ischemic attack. The neurological investigations were left to the neurologist discretion and systematically included brain MRI, cerebral arteries Doppler evaluation and the search for thrombophilia. Patients between 18 and 65 years of age could be included but in the absence of any cardiovascular risk factor above the age of 55. ASA was defined as an excursion of the atrial septum above 10 millimeters in the transoesophageal echocardiography (TEE) M-Mode11. The study was conducted between December 1st 1998 and August 31st 2003 and 40 consecutive patients were included. Chronologically, 20 patients were first treated by a PFO-Star (Cardia, Burnsville, MI) device. Then, 20 other patients received a Starflex occluder (NMT, Boston, MA). The study protocol was approved by the hospital ethical committee and written informed consent was obtained from all patients.
Intervention & follow-up: The procedure was performed under endotracheal general anesthesia. Aspirin 500 mg and clopidogrel 300 mg were prescribed the day before the intervention. An intravenous bolus of heparin (100 IU/kg) was given and a 12-F venous sheath was inserted by the femoral route. Device implantation was guided by biplane fluoroscopy and multiplane TEE. Post procedural antithrombotic treatment consisted of Aspirin 100 mg during 6 months associated with clopidogrel 75 mg for 3 months. Low risk antibiotic prophylaxis for endocarditis was recommended for 1 year. Transthoracic echocardiography with micro-bubbles testing at 1, 3, 6 months was performed. Micro-bubbles were obtained by mixing 10 cc of saline with 0.5 ml of air. Three antecubital vein contrast injections were performed at rest and during the Valsalva maneuver. The number of micro-bubbles in the left atrium during 5 cardiac cycles following opacification of the right atrium was taken into account. The shunt was considered as minor if 3 to 9 bubbles were counted, moderate between 10 and 30 bubbles and important over 30 bubbles. A neurological follow-up was performed simultaneously.
Study end points & statistics: The primary endpoint was complete PFO closure at 6 months as assessed by transthoracic contrast echocardiography. Secondary endpoints were major complications related to the procedure and clinical recurrence at 1 year follow-up. Both groups of patients were compared by statistical analysis using the Fisher’s exact test. Statistical significance was defined as a p-value below 0,05.

Fig. 1: A Starflex device with deployed umbrellas.

Fig. 2: Illustration of a PFO-Star device in deployed position.
Results
Patients: A total of 40 patients were included in the present study, consisting of 20 patients in each group. Baseline clinical characteristics were similar in both groups (Table 1). Morphological and functional aspects of the PFO (who were similar for both groups) are illustrated in Table 2. A higher proportion of ASA was observed in the Starflex group, albeit not significant. There was no difference in terms of pre-procedural right-to-left shunt degree.
Intervention & follow-up: Procedural details are shown in Table 3. Except for a slightly longer procedural time, no significant differences were observed. Five (13%) minor peri-procedural complications, equally distributed in both groups, were noted: 4 patients suffered transient supraventricular tachycardia (2 patients in each group) and 1 transient ST segment elevation was observed in the PFO-Star group. Spontaneous resolution without biomarker modification was noted.
Because of the study design, long term clinical follow-up, obtained in all patients, was significantly longer in the PFO-Star group. The mean follow-up time was 37 ± 19 months (PFO-Star group: 52 ± 14 months, Starflex group: 22 ± 7 months).
Two major complications occurred in the PFO-Star group : a thrombus on the right side of the device which was successfully managed by oral anticoagulation and a fistula between the right atria and the aorta which completely resolved at 18 months after intervention.
Study end points: Complete closure of the PFO was higher in the Starflex group (90% vs 50% for the PFO-Star group, p=0.001). Nevertheless, there was no neurological recurrence during clinical follow-up. Concerning any complication related to the procedure, there was no significant difference between both groups.
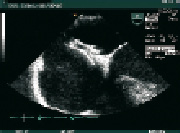
Fig. 3: Transoesophageal echocardiographic view of a Starflex device deployed through the interatrial septum.
Discussion
This single center prospective trial demonstrates that, independently of the acute procedural success, complete device closure of a PFO at 6 months follow-up is device dependent.
Despite the absence of randomized trials demonstrating its efficacy, percutaneous closure of PFO in patients suffering cryptogenic stroke is an emerging strategy. A large amount of clinical data on percutaneous closure of PFO is available11,14,16-18. In accordance with these data, the high percentage of ASA in our population did not preclude closure success17,19.
A first “head-to-head” comparison of PFO occlusion devices was already presented in one report20. Schwerzmann et al. allocated 100 patients (upon the discretion of the operator) with presumed paradoxical embolism in two equal groups treated either with a PFO-Star or Amplatzer (AGA Medical, Golden Valley, MN) device. Inclusion criteria were any patient with cryptogenic stroke and PFO. Implantation was performed without echocardiographic guidance and the primary end point was any symptom compatible with embolic recurrence during follow-up. Complete closure on follow-up was assessed by transoesophageal contrast echocardiography. Periprocedural complications were more frequent with the PFO-Star implant (16% versus 2% for the Amplatzer, p=0.01). At follow-up, a residual shunt was more frequent with the PFO-Star device (34% versus 6% for Amplatzer, p=0.004).
The present study differs in several ways from Schwerzmann’s work. Firstly, due to more stringent inclusion criteria, our study population is to be considered at higher risk for clinical recurrence. In particular, 68% of patients presented with ASA contrary to 25% in Schwerzmann’s series. Awaiting randomized evidence, our policy is to restrict PFO closure to this high risk population.
Secondly, implantation was echocardiography guided which may explain the higher procedural success rate despite a more challenging morphology. This is particularly important in this young patient population, where besides efficacy, safety is a key issue. No complications were encountered during PFO-Star placement in our series indicating the essential role of echocardiographic guidance.
Thirdly, complete closure was assessed by transthoracic rather than transoesophageal echocardiography. It is well known that the quality of the Valsalva maneuver is far better in a conscious patient (using transthoracic echography) than in a sedated patient (transoesophageal echography). Therefore, despite similar findings in both trials (indicating a significantly higher residual shunt with the PFO-Star device), residual shunting may be underscored in Schwerzmann’s observation.
The persistence of a significant shunt has been identified as a risk factor for embolic recurrence14,19,20. As a correlate, Windecker et al. recently demonstrated that complete closure of a PFO is superior to medical therapy in patients with cryptogenic stroke21. The inferior closure rate reported again with the PFO-Star device is unsatisfactory regarding to the potential risk of recurrent stroke14,19,20.
The present study has several limitations. Firstly, it is a small non-randomized trial. However, it has the advantage to present a homogeneous high risk group. Secondly, the present study was conducted with the devices that were available during the study period. Discrete modifications have been made in the design of the PFO-Star device which might change outcome measures if the trial was to be repeated at the present time.
In conclusion, complete percutaneous PFO closure in patients with presumed paradoxical embolism is occlusion device dependent. Acute procedural success does not implement late closure. This has important practical implications if PFO closure is planned in these patients.

