Abstract
Aims: To evaluate the long-term clinical outcome after drug-eluting stents (DES) implantation, and to test if patient selection could enhance their net clinical benefit.
Methods and results: We assessed the incidence of major adverse cardiac events (MACE=death, acute myocardial infarction, and target vessel revascularisation, TVR) and angiographic stent thrombosis (ST) during 3-year follow-up in a prospective multicentre registry. Propensity-score analysis to adjust for different clinical, angiographic and procedural characteristics was performed. Overall, 14,115 patients enrolled in the registry received solely BMS (n=9,565) or DES (n=4,550). The incidence of definite ST was 0.6% for BMS and 1.3% for DES (p=0.003). The propensity-score adjusted incidence of cardiac death and myocardial infarction was similar between the two groups (DES 11.9% vs. BMS 12.1%, HR 0.90, 95% CI 0.77-1.04), whereas DES were associated with lower rates of TVR (DES 11.6% vs. BMS 15.2%, HR 0.67, 95% CI 0.59-0.76). The efficacy of DES in reducing TVR increased with increasing likelihood of TVR at baseline.
Conclusions: The beneficial effect of DES in reducing new revascularisations compared to BMS extends out to three years without a significantly worse overall safety profile. The benefit seems more evident in patients with the highest baseline risk of clinical restenosis.
Introduction
Drug-eluting stents (DES) have been developed to reduce the incidence of restenosis and the need of new revascularisations following percutaneous coronary interventions (PCI).1-5 The initial enthusiasm raised by the extraordinary results of pivotal clinical trials, however, has been subsequently hampered by reports that DES might be associated with increased rate of late stent thrombosis and adverse clinical events over long-term follow-up.6-9
The immediate and obvious consequence of this concern was a diffuse, though inhomogeneous, reduction of DES utilisation. Besides, the health care providers and the regulatory bodies required more data to clarify the safety and effectiveness of DES use.10 Reassuring results came from extended follow-up of randomised controlled trials,11 as well as from patient-level metanalyses12,13 and registries.14-17 In this scenario, careful selection of patients who are candidate to receive a DES might be important, but at the moment, a clear indication about how to perform this selection is lacking. Strict adherence at labelled, evidence-based indications would be certainly appropriate, but this strategy is unable to offer solutions for most of the clinical situations encountered in daily practice.
With the present study, through the analysis of a large real-world multicentre registry, we sought to identify factors that may help selecting patients who are candidates to receive DES in order to enhance the long-term net clinical benefit of these devices.
Methods
Study design and patient population
The REAL registry (REgistro regionale AngiopLastiche dell’Emilia-Romagna) has been previously described.15,18,19 Briefly, it is a large prospective web-based registry launched on July 2002 and designed to collect clinical and angiographic data of all consecutive percutaneous coronary interventions (PCI) performed in a northern Italian region (4-million inhabitants). All thirteen public and private centres of interventional cardiology in the region participated in this data collection. Procedural data are retrieved directly and continuously from the resident databases of each laboratory, which share a common pre-specified dataset. These data are open for evaluation and periodic audits performed by the Regional Health Care administration. The aim of the registry was to monitor the introduction of DES in clinical practice, to evaluate profiles of utilisation and the relative clinical outcomes, although it has gradually become a permanent and useful tool for several different evaluations on PCI procedures performed in the region. Timing of follow-up is pre-specified (update every three months). A complete data analysis and the relative report are performed every year by the Regional Health Care agency. All other types of analyses can be proposed by the Steering Committee and by any investigator participating to the registry. In the last case, the plan of the analysis should be previously submitted and approved by the Steering Committee, which also establishes the priority of the analyses to be done.
Study flow is reported in Figure 1.
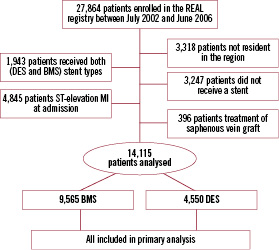
Figure 1. Study flow.
Between July 2002 and June 2006, 27,864 consecutive patients were enrolled in the registry. After exclusion of patients not resident in the region (n=3,318), patients with ST-elevation MI at admission (n=4,845), patients receiving both BMS and DES (n=1,943), patients not receiving any stent (n=3,247), and patients with treatment of saphenous vein grafts (n=346), 14,115 patients were considered for the present study.
The REAL registry was based on current clinical practice; therefore regulatory authorities required only an ordinary written informed consent to PCI and to data collection, which were obtained from all patients. The protocol of the study is in accordance with the Declaration of Helsinki.
Procedures and post-intervention medications
Interventional strategy and device utilisation including DES type were left to the discretion of the attending physicians. Periprocedural glycoprotein IIb/IIIa inhibitors and antithrombotic medications were used according to the operator’s decision and current guidelines. Antiplatelet treatment was prescribed according current standards of treatment, including lifelong aspirin to all patients, 1-month ticlopidine (250 mg bid) or clopidogrel treatment (75mg/d) for patients treated with BMS. Double antiplatelet treatment was extended to at least two months for patients treated with DES. Duration of dual antiplatelet therapy was gradually augmented for patients treated with DES during the four years of enrolment based on new recommendations. 20
Definitions and follow-up
The primary endpoint of the survey was the occurrence of major adverse cardiac events (MACE), defined as 1) death (cardiac and non-cardiac), 2) non-fatal acute myocardial infarction (AMI), and 3) target vessel revascularisation (TVR). Myocardial infarction during follow-up was diagnosed by local cardiologists at the hospital of admission according to standard criteria (rise in the creatine kinase level to more than twice the upper normal limit with an increased creatine kinase-MB, newly developed Q waves). Target vessel revascularisation was defined as any re-intervention (surgical or percutaneous) to treat a luminal stenosis occurring in the same coronary vessel treated at the index procedure, within and beyond the target lesion limits. The protocol of the REAL registry did not include routine angiography for any subgroup of patients, therefore virtually all reinterventions can be considered clinically-driven. Thrombotic stent occlusion was angiographically documented as a complete occlusion (TIMI flow 0 or 1) or a flow limiting thrombus (TIMI flow 1 or 2) of a previously successfully treated artery. Lesion length and vessel reference diameter were visually estimated by the operators. On-line quantitative coronary analysis was allowed if required by the attending physician. Follow-up was obtained directly and independently from the Emilia-Romagna Regional Health Care agency through the analysis of the hospital discharge records and the mortality registries. This process ensures complete follow-up for 100% of the patients resident in the region, including all out-of-hospital deaths (this is the reason for the a priori exclusion of patients who live outside the region). All repeat interventions during follow-up (either surgical or percutaneous) were prospectively collected from the single institutions as well and matched with the administrative data to adjust for eventual inconsistency. Hospital records were reviewed for additional information whenever deemed necessary. Specific queries were sent to the single institution to justify/correct discrepancies between administrative data, largely provided by independent cardiologists, and data derived from the web-based PCI database, compiled by the interventional cardiologists.
Statistical analysis
Continuous variables were expressed as mean±SD and were compared using Student’s unpaired t-test. Categorical variables were expressed as counts and percentages and chi-square test was used for comparison. The cumulative incidence of adverse events was estimated according to the Kaplan-Meier and compared by the log rank test. Because of the observed differences in baseline characteristics between the treatment groups, a propensity score analysis was carried out by use of a logistic regression model for treatment with DES versus BMS. This analysis included a number of clinical, angiographic and procedural variables, such as age, sex, diabetes mellitus, prior angioplasty, prior coronary artery bypass graft, prior myocardial infarction, neoplastic disease, chronic renal disease, heart failure, dementia, cerebrovascular disease, acute coronary syndrome at admission, left ventricular ejection fraction<35%, in-stent restenosis, target vessel, left main stenting, number of lesions treated, reference vessel diameter, lesion length, ostial lesion, chronic total occlusion, bifurcation, year and hospital of treatment. The logistic model by which the propensity score was estimated showed good predictive value (C-statistic=.826), and calibration characteristics by the Hosmer-Lemeshow test (p=.08). The score was then incorporated into subsequent proportional-hazards models as a covariate. Cox proportional hazards models were used to assess relative risk of adverse events in subgroups of patients.
To further investigate the impact of the baseline risk of restenosis on the clinical impact of DES, we performed a multivariable logistic regression analysis to identify the independent predictors of target vessel revascularisation at 1-year in patients treated with BMS, including all variables listed in Tables 1 and 2.
Variables were selected when p<.10. From the logit (p) = b0+b1*x1+b2*x2+…+ bn*xn=Xb estimated by logistic regression analysis, we calculated the function of probability that is calculated as follows: p = eXb/(1+eXb). This function allowed us to assign a baseline “probability” of TVR (i.e. risk) also to DES-treated patients. Subsequently, the entire population was subdivided into quintiles of risk, in order to identify the relative efficacy of DES vs. BMS within each quintile of risk. The number needed to treat (NNT), i.e. the number of patients to treat with DES instead of BMS in order to prevent 1 TVR event, was estimated with the standard function: NNT=1/absolute risk reduction. The absolute risk reduction is the difference between rates of event in the two treatment groups. All analyses were performed with the SAS 9.1 system.
Results
Between July 2002 and June 2006, 14,115 patients (21,035 lesions) enrolled in the REAL registry received solely BMS (n=9,565) or DES (n=4,550, 2,566 [56%] sirolimus- and 1,701 [37%] paclitaxel-eluting stent, 7% combined) to treat narrowings of the native coronary tree.
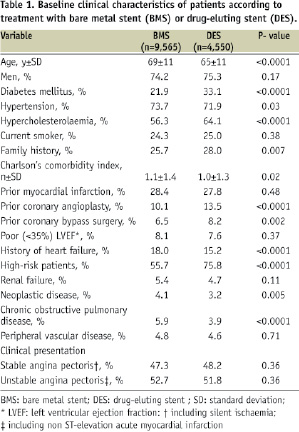
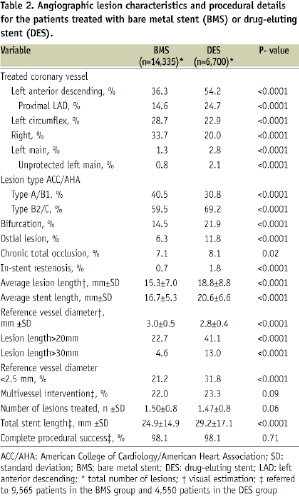
As reported in Table 1, the two populations showed several differences. Patients in the DES group were younger (65±11 years vs. 69±11 years, p<.0001), and more frequently had diabetes mellitus (33.1% vs. 21.9%, p<.0001), whilst patients in the BMS group had more comorbidities like neoplastic disease and chronic obstructive pulmonary disease, as confirmed also by the Charlson’s comorbidity index. Diagnosis of acute coronary syndrome at admission was similar in the two groups. As expected, DES were used in more complex lesions (Table 2), such as American Heart Association/American College of Cardiology type B2/C (69.2% DES vs. 59.5% BMS, p<0.0001), in-stent restenosis (1.8% vs. 0.7, p<.0001), bifurcations (21.9% vs. 14.5%, p<.0001), ostial lesions (11.8% vs. 6.3%, p<0.0001), long lesions (lesion length>20 mm: 41.1% DES vs. 22.7% BMS, p<0.0001), and small vessels (reference vessel diameter ≤ 2.5mm 31.8% vs. 21.2%, p<0.0001). Proximal left anterior descending coronary artery and unprotected left main coronary artery were also more frequently treated with DES. Complete procedural success was obtained in 98.1% of the procedures in both groups.
The 3-year unadjusted cumulative incidence of major adverse cardiac events is showed in Table 3.
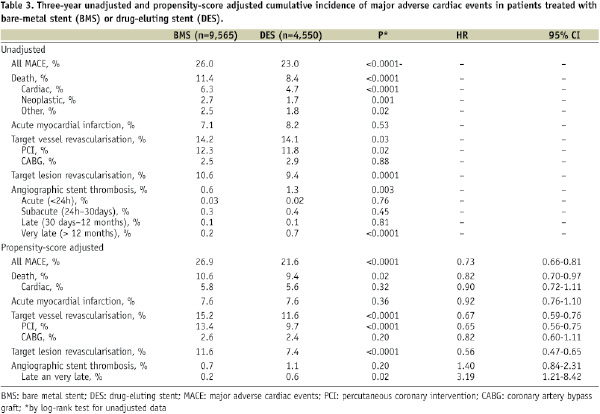
As showed in Figure 2, the propensity-score adjusted incidence of cardiac death and myocardial infarction was similar between the two groups, whilst DES were associated with significantly lower rates of TVR (DES 11.6% vs. BMS 15.2%, HR 0.67, 95% CI 0.59-0.76), and MACE (DES 21.6% vs. BMS 26.9%, HR 0.73, 95% CI 0.66-0.81).
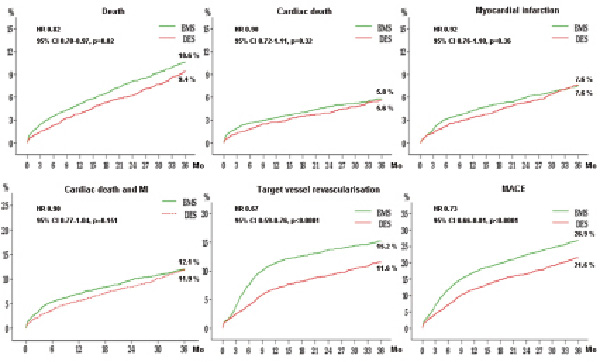
Figure 2. Propensity score-adjusted clinical outcome in bare metal stent (BMS) and drug-eluting stent (DES) groups.
The incidence of definite stent thrombosis was 0.6% for BMS and 1.3% for DES (p=0.003 by log rank test), where the excess of such events for DES took place beyond 12 months (Figure 3).
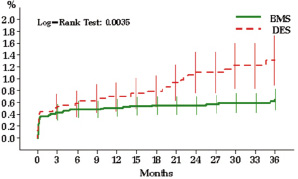
Figure 3. Incidence and timing of definite stent thrombosis. Bars denote confidence intervals.
The propensity-score adjusted comparison between different stent types is illustrated in Figure 4.
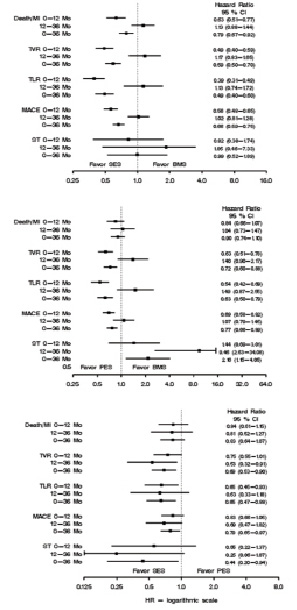
Figure 4. Clinical outcome for sirolimus- (SES) and paclitaxel-eluting stent (PES).
Both SES and PES hold an advantage over BMS in terms of reduction of TLR, TVR and MACE, which is largely gained during the first year and then partially lost thereafter. As compared to PES, the SES presented significantly lower risk of TVR (HR 0.69; 95% CI 0.53-0.90), TLR (HR 0.65; 95% CI 0.47-0.88), MACE (HR 0.79; 95% CI 0.65-0-97), and stent thrombosis (HR 0.44; 95% CI 0-20-0.94).
Subsequently, we divided our population into quintiles based on the likelihood of 1-year TVR (Table 4), as previously described.
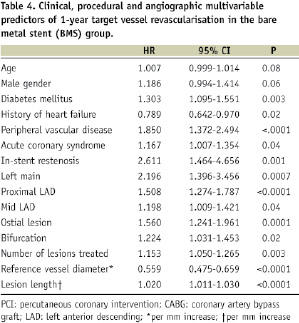
Relative risk associated to DES vs. BMS utilisation in each quintile is showed in Table 5.
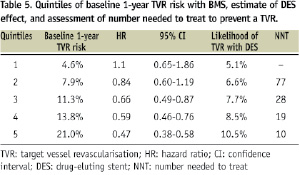
We observed that the relative efficacy of DES in reducing TVR rates increased with increasing likelihood of TVR at baseline, and this reduction was statistically significant only for the three highest quintiles of risk (i.e., when the baseline probability of TVR was >11%). Table 6 reports the 3-year propensity-score adjusted clinical outcome according to quintiles of baseline 1-year TVR risk. Figure 5 illustrates the landmark analyses for TVR relative to the combined first and second quintiles of patients versus the combined third, forth and fifth quintiles and the correspondent definite stent thrombosis rates over three years.
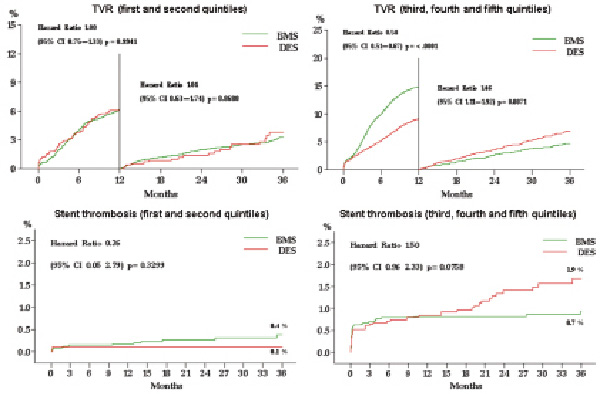
Figure 5. Landmark analyses of target vessel revascularisation and stent thrombosis rates for DES and BMS-treated patients for the two lowest and the three highest quintiles of risk of TVR.
There was no significant difference in terms of cardiac death and MI between these last two groups (Figure 6), but a trend toward reduced incidence of the combined endpoint in the highest risk group was observed.
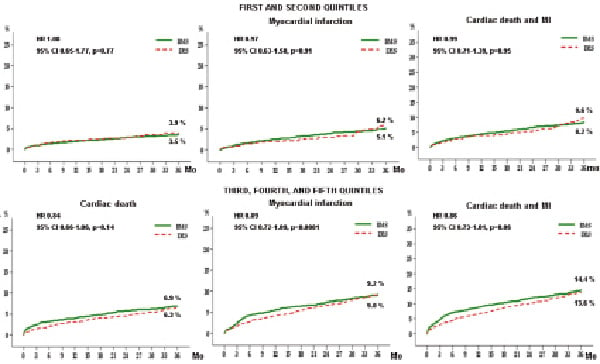
Figure 6. Propensity score-adjusted rates of hard clinical endpoints in bare metal stent (BMS) and drug-eluting stent (DES) groups for the two lowest versus the three highest quintiles of risk of target vessel revascularisation.
Discussion
The principal findings of this large multicentre registry of PCI include the following: 1) use of first-generation DES was associated with reduced 3-year incidence of TVR and MACE as compared to BMS; 2) the incidence of definite stent thrombosis with DES after the first year is low, but significantly higher than BMS, although rates of cardiac death and myocardial infarction remained similar between the two groups; 3) use of DES in patients at higher baseline risk of restenosis is the most effective strategy to reduce TVR; 4) different DES systems are associated with different clinical outcome.
Several studies have shown a significant and sustained reduction in the need for repeat coronary revascularisation procedures associated to DES in comparison to BMS. Our study confirmed a persistent 33% reduction of TVR up to three years associated with DES utilisation in a heterogeneous and complex patient population. This is remarkable because, in general, DES were used to treat more diffuse and possibly aggressive disease. Hence, the true benefit of DES may be somewhat underestimated by TVR rates, even if all analyses were adjusted for measurable baseline factors and notwithstanding that the absolute difference in TLR rates (which better describe the true effect of DES) between DES and BMS was not very dissimilar from that observed in TVR rates. On the other side, DES may engender adverse arterial responses, including delayed endothelialisation, impaired vascular healing, and hypersensitivity to the polymeric coating that altogether may predispose to stent thrombosis.21,22 Indeed, very late thrombosis (i.e. >12 months after the procedure) is a phenomenon virtually absent with BMS, whereas it has emerged as an important drawback for DES.6-9 Albeit infrequent, stent thrombosis is often associated to catastrophic clinical consequences such as myocardial infarction and sudden cardiac death, therefore its prevention deserves the greatest attention.23-25 In a previous report from the REAL registry,15 we did not observe a significantly different incidence of stent thrombosis (according to the Academic Research Consortium definitions)26 between DES and BMS over two years of follow-up. In the present study, with larger population and longer follow-up, the incidence of definite stent thrombosis after DES, though numerically low, was twice as high as BMS (1.3% vs. .6%, respectively), and the excess of these events took place clearly beyond 12 months. Nevertheless, rates of cardiac death and myocardial infarction were very similar between DES and BMS, confirming previous reports about the overall long-term safety of DES. A troublesome observation is that in our study the risk of stent thrombosis seems to parallel the risk of restenosis, thus meaning that DES benefits and DES drawbacks somehow match up in the same patients. This association is generally accepted for acute and subacute thrombosis, but it is less clear for late thrombosis14,27. On the other hand, rates of cardiac death and MI remained similar between BMS and DES also in higher risk groups (Table 6 and Figure 6), and hard events were even significantly lower in the highest risk quintile. Taken together, these findings exhort to pay the greatest attention to procedural (e.g., optimal stent expansion) and pharmacological details (i.e. compliance and length of dual antiplatelet therapy) in higher risk patients, in order to minimise the incidence of DES thrombosis and further improve clinical results.
The decision-making about the optimal PCI strategy should take into account several patient-related factors that might influence the choice between BMS and DES. In this context, physicians should investigate and carefully consider in advance the likelihood of patient compliance to prolonged antiplatelet therapy. Because early discontinuation of dual antiplatelet therapy is a major determinant of DES thrombosis,23,28,29 the presence of clinical conditions predisposing to thrombosis must be ruled out (e.g. essential thrombocythemia, antiphospholipid syndrome, lupus anticoagulant). The choice of the most appropriate treatment usually derives from the trade off between the predicted benefit and the risk associated to the treatment itself. Hence, the choice to implant a DES should take into account the balance between the risk of restenosis and the incremental risk of thrombosis associated to DES. Garg et al30 developed a Markov model comparing DES versus BMS over a range of incremental risk and duration of risk of very late stent thrombosis. They suggested that the threshold excess risk of very late DES thrombosis compared with BMS, above which BMS would be the preferred strategy, is 0.14% per year over four years of follow-up. Under this perspective, the 0.6% excess late stent thrombosis rate per year (up to three years) reported by some authors is worrisome.14 On the other hand, the stent thrombosis threshold to prefer DES over BMS increased as the population risk of restenosis increased and decreased as the vulnerable time window lengthened.30 How can we explain this behaviour? Recently, it has been unravelled that BMS restenosis is not a benign entity as it was thought in the past,31 and target lesion revascularisation events are associated with a low, but finite, incidence of periprocedural death or myocardial infarction.32 Consequently, the prevention of restenosis-related adverse events by DES might offset some or all of the excess risk derived from stent thrombosis.32 It is reasonable to hold that the net clinical benefit of DES must be weighted, not only toward stent thrombosis, but also against the most comprehensive and important endpoint of combined hard adverse events, i.e. MI and death. In this scenario, development of models for selecting patients who are most likely to derive a net clinical benefit from DES instead of BMS could be the best strategy. Our study is a first attempt to move a step in this direction. We hypothesised that DES utilisation might be more effective in patients at higher risk of restenosis, and the results of this study support this hypothesis. This is consistent, for example, with the results of the Ontario registry. In that experience, DES were associated with significant reductions in the rate of TVR among patients with two or three risk factors for restenosis (i.e., diabetes, small vessels, and long lesions) but not among lower-risk patients.17
A possible criticism to this approach is that most of the high-risk patients would be “off-label” for DES both in Europe and in the US. However, previous studies have demonstrated that use of drug-eluting stents for «off-label» indications was indeed associated with worse outcomes at two years than on-label application, but it was also associated to better clinical results than in a comparable group of patients treated with BMS.33 Although not conclusive, these data should be reassuring for the interventional cardiology community. Certainly, they suggest that case selection and physician decision making regarding use of DES was safe and appropriate.
Importantly, DES may differ one from the other in all components: stent platform, polymer carrier, and drug. This makes very unlikely a uniform class effect. Consistently with several other reports, in this study we observed significantly lower rates of new revascularisations and stent thrombosis with the SES as compared to the PES,12,14,19 which is another element that should be taken into account for stent selection.
The good results obtained with DES in our registry may be partially attributed to a different pharmacotherapy with respect to BMS. The requirement of an extended course of clopidogrel therapy with DES is now generally accepted, although currently available data are insufficient to support a specific duration of dual antiplatelet therapy for all patients.34 On the other hand, 12 to 18 months of clopidogrel are usually recommended to patients with acute coronary syndromes (i.e. around 50% of our population), including those receiving BMS. Further, it must be remembered that along with favourable effects, dual antiplatelet treatment can increase the risk of severe bleeding events.35 The ability to differentiate classes of risk for ST could help identification of patients in which longer dual antiplatelet therapy might be more beneficial.
Advances in stent platforms, polymer carriers and drugs are being evaluated to enhance long-term safety and to reduce the risk of late ST associated to DES. However, at the present time, there are no solid data to affirm superior safety of any new device over the so called first-generation DES.
Limitations
This study suffers all the inherent limitations of observational studies. The time period of combined antiplatelet therapy was variable, and we observed a progressive increase over time, but no specific information is available in the registry. The logistics and the costs of contacting periodically every patient to collect information on pharmacologic modifications during follow-up and compliance to prescription are virtually prohibitive for registries like this (at the moment, around 40,000 patients enrolled). In this study, we report only definite stent thrombosis rate. Although information on probable and possible thrombosis episodes would be important to better understand the phenomenon of DES-related late stent thrombosis, the ultimate objectives of safety evaluations remains death and MI and, in this perspective, this limitation could be considered less relevant.
Conclusion
The REAL registry demonstrates long-term safety and efficacy of first-generation DES, and showed that DES are more effective than BMS in reducing the need for target-vessel revascularisation and major adverse cardiac events especially in patients at highest baseline risk for restenosis. The observed small excess of definite very late stent thrombosis seems to be outweighed by the reduction of restenosis-related adverse events. DES implantation must be carefully considered on an individual patient basis. Development of models to identify more precisely patients who are best candidates for DES implantation might increase the net clinical benefit of these devices. The present scenario could be changed by the introduction in clinical practice of new drug-eluting stents.
Appendix
The Emilia-Romagna REAL (REgistro Regionale AngiopLastiche) Investigators
Study coordination and statistical analyses:
Antonio Marzocchi, Francesco Saia (Istituto di Cardiologia, Università di Bologna, Policlinico S. Orsola-Malpighi);
Roberto Grilli, Paolo Guastaroba, Elena Berti (Agenzia Sanitaria Regionale Emilia-Romagna, Bologna).
REAL investigators
Istituto di Cardiologia, Università di Bologna, Policlinico S. Orsola-Malpighi: Antonio Marzocchi, Francesco Saia, Cinzia Marrozzini, Paolo Ortolani, Tullio Palmerini; Unità Operativa di Cardiologia, Ospedale degli Infermi, Rimini: Giancarlo Piovaccari, Andrea Santarelli, Domenico Santoro, Nicoletta Franco; Unità Operativa di Cardiologia Interventistica, Ospedale S. Maria Nuova, Reggio Emilia: Antonio Manari, Paola Giacometti, Stefano Fioroni, Vincenzo Guiducci, Gianluca Pignatelli; Divisione di Cardiologia, Ospedale Maggiore, Parma: Luigi Vignali, Alberto Menozzi, Maria Alberta Cattabiani, Emilia Solinas; Laboratorio di Emodinamica, Hesperia Hospital, Modena: Alberto Benassi, Giuseppe D’Anniballe, Luigi Steffanon; Divisione di Cardiologia, Ospedale Maggiore, Bologna: Pietro Sangiorgio, Gianni Casella, Andrea Rubboli, Giampiero Nobile; Unità Operativa di Cardiologia - Centro Interventistico, Sezione di Emodinamica - Aritmologia «B. Venturi», Ospedale S. Maria delle Croci, Ravenna: Aleardo Maresta, Elisabetta Varani, Marco Balducelli, Giuseppe Vecchi, Matteo Aquilina; Ospedale di Piacenza: Alessandro Capucci, Francesco Passerini, Gabriella Giovannini, Maria Alberta Cattabiani, Carlo Tumscitz; Laboratorio di Emodinamica, Ospedale Morgagni di Forlì: Fabio Tarantino, Ottorino Catapano, Filippo Ottani, Marcello Galvani; Laboratorio di Emodinamica, Policlinico di Modena: Fabio Sgura, Rosario Rossi; Modulo Dipartimentale di Emodinamica di Ferrara: Gianfranco Percoco, Fabrizio Ferrari, Marco Valgimigli, Gianluca Campo; Unità di Cardio-Angiologia Interventistica, Casa di Cura Villa Maria Cecilia Hospital, Cotignola (RA): Alberto Cremonesi, Fausto Castriota, Enrico Ricci, Raffaella Manetti, Armando Liso, Kareem Oshoala; Laboratorio di Emodinamica, Nuovo Ospedale S. Agostino, Modena: Stefano Tondi, Paolo Magnavacchi, Domenico Tosoni, Carlo Cappelli.
Funding sources
This study was supported by the Regional Health Care Agency of Emilia-Romagna, Bologna, Italy.

