
The 2018 EAPCI summit was held in Sophia Antipolis, France, on 20 and 21 June, with the participation of delegates and presidents from more than 20 national cardiac societies and working groups of interventional cardiology. This summit was dedicated to the new EAPCI initiative “patient focus in cardiovascular interventions”. The objective of this programme is to address patients’ needs through engagement and advocacy, for better access to data and information relevant to patients in need of cardiovascular interventions. By capturing the patients’ experience, the ambition of the summit was to understand patients’ interactions with interventional cardiologists better in order to determine the degree to which their needs are met and, where applicable, to project improvements.
Patient-reported experience metrics (PREMs)
The summit was organised into four workshops, four lectures and one symposium. The first workshop focused on patient-reported experience metrics (PREMs)1. The importance of developing PREMs is underscored by the fact that capturing patient experiences may: a) help to monitor the quality and safety of healthcare; b) contribute to the development and improvement of services; and c) enable benchmarking of performance and the monitoring of the effectiveness of interventions. Performing a survey among the delegates it became clear that PREMs are seldom collected, other than by regulatory bodies in a few cases2-4. Difficulty in interpreting the patients’ perspectives was reported as the major challenge in developing and implementing effective PREMs. Nevertheless, the delegates performed a very useful exercise by putting themselves in the shoes of their patients, reporting what they believe would be relevant when a patient approaches the healthcare system (Figure 1).

Figure 1. PREMs proposed by the delegates on the flipcharts during workshop 1.
Guidelines and registries
The European Society of Cardiology (ESC) is taking concrete steps to break down barriers to optimal delivery of cardiovascular care. In his first lecture, Marco Roffi convincingly explained that one of the important steps towards better quality of cardiovascular care within the EU was to improve the penetration and implementation of the ESC guidelines. Major efforts by the Clinical Practice Guidelines (CPG) Committee and by the Guidelines Task Forces have been directed towards shortening the number of pages in the upcoming guideline documents, improving the presentation by summarising key messages in tables and “catchy figures”, by listing “dos and don’ts”. In collaboration with the national cardiac societies, guideline summaries in local national languages should improve penetration of standards of cardiovascular management and care. Monitoring the implementation of guidelines represents an important task of the EURObservational research programme (EORP). During the symposium, discussion took place on the importance of mapping the status of the European healthcare systems from a cardiology perspective, in order to monitor trends, disparities, gaps and associations between fundamental variables. The ESC Atlas of Cardiology represents a unique data set containing a plethora of descriptive and quantitative data for each of the 56 ESC member countries5. From a strategic partnership between the ESC Atlas Health Policy Unit led by Panos Vardas and the EAPCI, the first edition of the EAPCI White Book was presented during the symposium. The EAPCI White Book represents an important effort in the direction of a systematic mapping of interventional cardiology practice collected from 16 national cardiac societies and working groups of interventional cardiology (Figure 2). Finally, the CPG Committee is also evaluating the possibility of patient engagement in the guideline drafting/reviewing process in an attempt to include patients’ perspectives regarding the quality of cardiovascular medicine. This CPG Committee action is to be commended and should become the rule for future guidelines as it is in line with one of the strategic objectives of the ESC, namely to launch the patient’s engagement initiative, as illustrated by Donna Fitzsimons in the second lecture (Figure 3). Specifically, PREMs refer to the effort to inform patients as much as possible about all relevant aspects of quality cardiovascular care including a balanced presentation of options of treatment, the need for and possibilities of rehabilitation, and all other aspects of the healthcare system supporting them. Patient engagement involves “providers and patients working together to improve health”, as quoted from a report of the Healthcare Information and Management Systems Society. The patients and physicians are seen as partners with the goal of achieving better patient care while meeting the needs and expectations of all stakeholders involved. The ESC and EAPCI are committed to building and developing the ESC patient forum, and intend to thread patient engagement into all our activities.
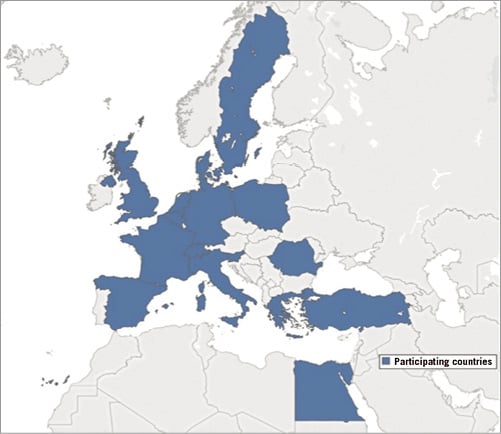
Figure 2. National cardiac societies participating in the 2018 edition of the EAPCI White Book: Belgium, Denmark, Egypt, France, Germany, Greece, Italy, the Netherlands, Poland, Romania, Slovenia, Spain, Sweden, Switzerland, Turkey, United Kingdom. Data collected were on the following topics: a) healthcare resources with a focus on interventional cardiology; b) procedures and resources for percutaneous coronary interventions; c) procedures and resources for structural heart interventions.
Figure 3. Opportunities deriving from the engagement of patients in all relevant aspects of quality cardiovascular care.
Patient engagement and advocacy
The second workshop was dedicated to the informed consent (IC) process concerning patients undergoing invasive coronary interventions. IC should be mandatory as it represents a legal requirement, and it is a key element of patient involvement, implying a correct understanding of the risks, benefits, and caveats of the procedure being performed. Personnel in charge of obtaining the IC should also be clearly defined and possibly be represented by a third party not directly involved in the proposed procedure in order to prevent any possible conflict of interest. Nevertheless, the delegates reported a significant heterogeneity in the IC process for patients with stable coronary artery disease, e.g., a written IC is not mandatory in some countries; very seldom are events organised to inform patients about procedures and, when present, these campaigns are organised by companies; only in a few cases can the patient learn about operator/team experience and complication rates before undergoing a procedure. Patient-team connection after the procedure is in most cases through direct contact with the treating physician; in a few cases this connection is institutionalised via a telephone link with the hospital. The consensus of this workshop was the need to prepare audiovisual tools to standardise and facilitate patient understanding of the procedure/treatment being performed/applied. Obtaining IC should also be standardised and documented digitally.
In the third lecture, Robert Byrne highlighted that advocacy represents one of the five strategic pillars of the ESC. Advocacy means arguing for a cause in order to influence decisions within political, economic or social systems. The ESC advocacy actions for the patient’s safety are listed as follows:
1. Education and training of physicians as advocates and experts for the provision of advice in relation to regulatory affairs.
2. Engagement with regulators to improve the safety of approved medical devices, especially when associated with high risk and implantable.
3. Increasing awareness of physicians regarding the importance of reporting of serious adverse device events for improving patient safety.
4. Engagement with health technology assessment agencies to ensure access to approved medical devices in an equitable manner.
The institution of morbidity and mortality conferences on a regular basis to review cases and complications might represent an additional action to be implemented. The advocacy’s strategic outcomes are to position the ESC as a valid source of unbiased scientific information, to provide readily available expert ESC spokespersons for relevant stakeholders, and to create a favourable political and regulatory environment for the promotion and protection of cardiovascular health.
The third workshop was dedicated to patient involvement in clinical research. Patient-specific (older age, out-of-state residence and female sex), and trial-related (longer trial duration and intensive trial-related testing) factors associated with not participating in cardiovascular trials were identified previously6. Additional existing challenges were identified before the study in the notable disparity between countries where patient involvement is mandatory (e.g., for grant application) and the rest of the world. During the study there is a different way to structure the informed consent process, spanning from no consent (e.g., in ethically approved studies), to a postponed or standard consent process. After the study, the way to disseminate the results is also different, spanning from “nothing” to institutional websites with study results in lay language. The delegates agreed on key action points and deliverables. Before a study, patients should be involved in the prioritisation of research ideas and review of research projects; patient-reported outcome metrics (PROMs) should be defined and validated in the cardiovascular field (in general, they are underused); more information directed to patients should be given regarding how to participate in clinical trials. During a study, there is a need to improve the clarity and transparency of the informed consent process, to involve patients in the review process and in ethical committees, and to appreciate that there are different ways of getting the consent depending on the clinical scenarios. After a study, it is important to collect feedback on the experience and satisfaction of patients at the end of the trial, and to inform the patients and the community regarding the study results.
EAPCI initiatives
The EAPCI has promoted several initiatives where patient involvement represents the key element. Dariusz Dudek focused on the Valve For Life programme in Poland, an important EAPCI initiative focusing on addressing the disparity in access to percutaneous transcatheter aortic valve implantation. In those countries where Valve For Life has been initiated, communication via public campaign (media initiatives, social media, educational movies, medical events) was shown to be a successful tool to improve public awareness and patient involvement. This was associated with an increase in therapy adoption (up 30%) simply because this was requested by the patients themselves, with no changes in reimbursement policy. Patients’ engagement further facilitated ongoing discussion with government representatives and decision-making bodies.
The last workshop focused on how to improve patient access to therapies for aortic stenosis and ST-elevation myocardial infarction. The challenge in the clinical management of patients with aortic stenosis relates to the large heterogeneity of transcatheter aortic valve implantation (TAVI) adoption among European countries and within the same country, mainly related to the reimbursement system. How should this be addressed? Mainly by informing the decision makers about objective treatments and relevant data, and by identifying dedicated resources (e.g., innovation funds). A scientific society can help to influence the stakeholders by the advocacy of dialogues with the regulatory bodies and healthcare insurance companies. Additional actions to help improvement of TAVI adoption are in increasing patient awareness (such as the Valve For Life Poland) and the active involvement of the cardiac surgeons.
In patients with STEMI, the Stent, Save A Life, another important EAPCI initiative, improved overall (>90%) primary PCI (pPCI) adoption, yet some countries are still below these targets. In addition, the way pPCI is performed can be heterogeneous (e.g., contact to balloon time, etc.). Common reasons preventing STEMI patients from accessing (timely) pPCI are: a) lack of a STEMI network; b) patient self-presenting to the wrong hospital, ambulance transfer time from one hospital to another, city traffic, etc.; c) patient awareness – the need to have an iterative public campaign. How should this be addressed? By identifying risk groups to be targeted by specific awareness campaigns (e.g., diabetic patients), by involving general practitioners in the awareness campaign, and by allocating more resources to the treatment of STEMI patients.
Expected outcome metrics
Successful implementation of the consensus points of the EAPCI summit can be monitored through the following outcome metrics:
– Development and implementation of patient-reported experience metrics (PREMs) and patient-reported outcome metrics (PROMs) in cardiovascular interventions.
– Patient engagement in the review process of guidelines.
– A uniform IC process improved, standardised and implemented overall.
Conclusions
The 2018 EAPCI summit focused on the unmet clinical needs of patients in interventional cardiology. Delegates reached a consensus on the importance of developing PREMs and PROMs as important tools to improve clinical practice, i.e., knowing what patients think of their healthcare providers would help to identify neglected issues. Patient engagement was underscored as a key step to improving therapy compliance and participation in research protocols. In this regard, a consensus was reached to promote a standardised informed consent process that would be readily available to all EU patients, for example by means of audiovisual tools. Finally, the EAPCI stands strongly by the ESC in promoting patient advocacy initiatives aimed at improving patient access to novel and more efficacious therapies in all EU regions.
Acknowledgements
The important contribution to the 2018 EAPCI summit of the following delegates is acknowledged: Magdy Abdelhamid (Egypt), Tahsin Al Kinani (Iraq), Joao António Brum Silveira (Portugal), Israel Barbash (Israel), Daniel Blackman (UK), Salome Coelho (Portugal), Jaan Eha (Estonia), Vladimir Ganyukov (Russian Federation), Lene Kloevgaard (Denmark), Jorgo Kostov (Republic of North Macedonia), Peter Ludman (UK), Deyan Nenov (Bulgaria), Zsolt Piroth (Hungary), Ion Popovici (Republic of Moldova), Tuomas Rissanen (Finland), Matej Samos (Slovakia), Giulio Stefanini (Italy), Giuseppe Tarantini (Italy), Ibrahim Terzic (Bosnia and Herzegovina), Hans-Henrik Tilsted (Denmark), Ramunas Unikas (Lithuania), Niels Van Royen (Netherlands), Nils Witt (Sweden), Wocjciech Wojakowski (Poland), Samir Ztot (Morocco).
Conflict of interest statement
The authors have no conflicts of interest to declare.

