Introduction
The 3rd EAPCI Summit was dedicated to “Mapping interventional cardiology in Europe” (see full report in the Online Appendix). The main objective of this Summit was to reactivate the annual survey on interventional cardiology practice in the ESC countries in order to: a) monitor the heterogeneity in practices; b) disclose obstacles to guideline implementation; c) record the dynamics of harmonisation in interventional cardiology practices.
Workshop 1. Existing registries from national working groups
The experiences of data collection and registries in interventional cardiology from ESC countries were presented. A large heterogeneity in data collection exists, with very few countries having well-structured patient-level data collection. Most of the countries present only have centre-level data collection, while a significant number of countries have no structured data collection at all. The data are often collected on a voluntary basis, and in most cases entered by the local sites. In a few cases they are sent to a national coordinator or data manager responsible for entering the data into the registry. Quality control of data collection is very limited and consists of a clinical events committee or audits, performed in most cases on a limited random sample. All of the countries present were positive in terms of sharing data with the EAPCI and were open to a possible extension of data collected at national level.
Workshop 2. Building a common survey
On an individual basis participants were requested to select the minimum number of queries possible on four main topics: a) demographic and organisation; b) coronary interventions; c) peripheral interventions; d) structural heart interventions. After having completed the questionnaires individually, the participants were also requested to reach a group consensus on each of the four topics. Figure 1 shows the individual preference of the participants on the four topics. Figure 2 shows the results of the group consensus. There was a consensus for not including queries on peripheral interventions in this early phase, due to the large heterogeneity of professionals performing these procedures and the pattern of data collection. Overall, the minimum number of queries deemed interesting/important for an EAPCI survey by the majority of participants (>50%) was, on average, 65 chosen on an individual basis and 64 chosen on the basis of the group consensus (from a total of 115 proposed).
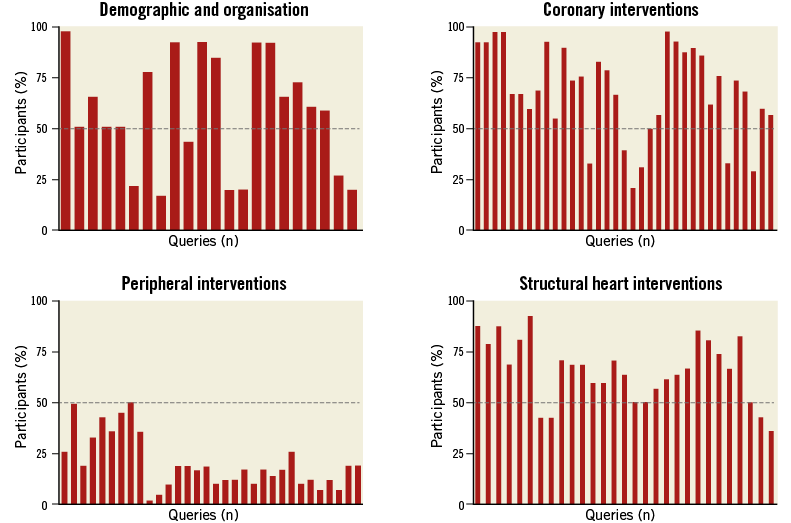
Figure 1. Individual preference for queries on the four main topics.
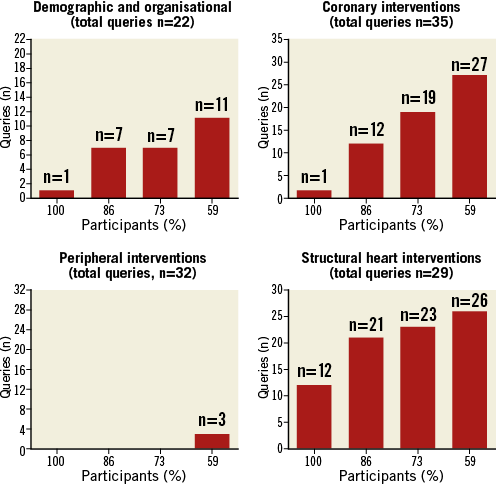
Figure 2. Group consensus on the four main topics.
Workshop 3. Challenges and solutions in building a common survey
The majority of participants agreed on adopting a mixed model of survey consisting of: a) requesting aggregated country data from the national cardiac societies or working groups running well-structured registries; b) requesting, via annual questionnaires, aggregated centre-level data from the national working group representatives from the other countries.
Workshop 4. Deliverables of the survey
The results of the EAPCI survey thus constituted may be the object of multiple publication strategies. These data can be published in a time-sensitive fashion on the EAPCI website, or reported in newsletters, national or international journals. The results can be communicated both to national and to international meetings. The publication policy should be discussed openly and agreed upon at the time the working groups are asked to share their data.
Conclusion
The 3rd EAPCI Summit has created the basis for the reactivation of the annual EAPCI survey on interventional cardiology practice in Europe. This was possible thanks to the active participation of numerous representatives of national Working Groups of Interventional Cardiology from the 56 ESC countries.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Appendix . List of participants and complete reports of the workshops.
LIST OF PARTICIPANTS FROM THE ESC COUNTRIES REPRESENTED IN ALPHABETICAL ORDER (http://www.escardio.org/membership/national-societies/Pages/welcome.aspx)
Alexander Alexandrov (Bulgaria), Emanuele Barbato (Belgium), Andreas Baumbach (UK), Sergio Berti (Italy), Marc Claeys (Belgium), Paul Cummins (The Netherlands), Dariusz Dudek (Poland), Jaan Eha (Estonia), Andrejs Erglis (Latvia), Giovanni Esposito (Italy), Habib Gamra (Tunisia), Vladimir Ganuykov (Russia), Omer Goktekin (Turkey), Michael Haude (Germany), Erkki Ilveskoski (Finland), Petr Kala (Czech Republic), Tuomas Kiviniemi (Finland), Lene Kloevgaard (Denmark), Rene Koning (France), Jorgo Kostov (Macedonia), Peter Ludman (UK), Eduard Margetic (Croatia), Josepa Mauri (Spain), Pascal Meier (Switzerland), Stefan Mot (Romania), Darren Mylotte (Ireland), Andrzej Ochala (Poland), Helder Pereira (Portugal), Sonia Petronio (Italy), Marin Postu (Romania), Svein Rotevatn (Norway), Zoltén Ruzsa (Hungary), Eugenio Stabile (Italy), Josef Stasek (Czech Republic), Christian Terkensen (Denmark), Ibrahim Terzic (Bosnia & Herzegovina), Hans Henrik Tilsted (Denmark), Ramiro Trillo (Spain), Ramunas Unikas (Lithuania), Rene Van Der Schaaf (The Netherlands), Franz Weidinger (Austria), Stephan Windecker (Switzerland), Adam Witkowski (Poland), David Zughaft (Sweden)
COMPLETE REPORT OF THE FOUR WORKSHOPS
Workshop 1. Existing registries from national working groups
The 3rd EAPCI Summit was dedicated to “Mapping interventional cardiology in Europe”. The main objective of this Summit was to reactivate the annual collection of information on interventional cardiology practice in the ESC countries in order to: a) monitor the heterogeneity in practices; b) disclose obstacles to guideline implementation; c) record the dynamics of harmonisation in interventional cardiology practices.
During the first workshop the experiences of data collection in 10 ESC countries were presented with representatives from four European macro-areas (Northern, Southern, Western and Eastern Europe).
In preparing their lectures, the representatives of the working groups were requested to address the following questions: a) name of the national registry of percutaneous procedures with URL website if available; b) number of centres participating in the registry; c) starting date of inclusion in the registry; d) modalities of data entry in the registry; e) availability to share part of the data with EAPCI ESC; f) availability to collect some additional data not included in the existing database; g) whether data generated by the national registry were published in peer-reviewed journals or local journals; h) kind of data gathered for each patient; i) availability to activate a registry in case of lack of an existing one.
From the presentations of the 10 ESC countries and the discussion with the other country representatives participating in the Summit, the following points were highlighted.
There is a large heterogeneity in data collection, from countries with centre-level data collection to countries with no structured data collection, with very few countries having well-structured patient-level data collection.
In most cases data are collected on a voluntary basis.
The best data collection is driven by reimbursement policies. In general, these data focused on new technologies and therapies (e.g., TAVI or BRS).
Registries available are web-based in only a few countries.
There is heterogeneity in the kind of data collected: very few countries have a complete data set on adult cardiovascular interventions (from coronary, peripheral to structural heart interventions), while very often registries are devoted to specific topics (STEMI registry, BRS registry, TAVI registry, etc.).
The data in the national registries are in most cases entered by the local sites (either at patient level or aggregated), whilst in a few cases they are sent to a national coordinator or data manager who is responsible for entering the data into the registry.
Ownership of the data belongs in most cases to the national working groups or scientific societies and is coordinated by the respective presidents. In a few cases, there is an independent data manager.
Quality control of data collection is very limited and consists of clinical events committees or audits performed, in most cases, on a limited random sample.
In general, aggregated data are collected in the vast majority of the centres within the countries. The centres not providing data are often small-volume or private centres.
The time delay to collect and/or validate the data is in the range of three to eight months.
All countries present were positive concerning the request to share the data with EAPCI and open to a possible extension of data collected at national level.
The important issue of the jurisdiction of data and the legal obligation of the informed consent of the patients was also discussed. There was consensus that the working groups and/or national societies remain the owners of the data and that they will be involved in any decisions on how to use these data. The patients provided their informed consent to share their data within the national registry, but not to a European registry. This issue could be overcome by collecting anonymous aggregated data from the centre and/or the country.
Workshop 2. Building a common survey
The participants of the EAPCI Summit were given four questionnaires with the task of selecting, from multiple possible items, those items of interest for constituting the survey.
In order to be representative of the different geographic areas, seven round tables were organised, each with an equal distribution of participants from Northern, Eastern, Southern, and Western Europe.
Firstly, and on an individual basis, the participants were requested to select the minimal number of queries possible on four main topics: a) demographic and organisation items; b) coronary interventions; c) peripheral interventions; d) structural heart interventions.
After having completed the questionnaires individually, participants were then requested to reach a group consensus within every round table on each of the four topics. After the group consensus, there followed an open discussion involving all of the participants.
Figure 1 shows the individual preferences of the participants on the four topics. For the demographic and organisational topic: 15 out of 22 queries were chosen by the majority of participants (>50%). For coronary interventions: 28 queries out of 35 were chosen by the majority of participants. In terms of peripheral interventions, none of the 32 queries was preferred by the majority of participants. Concerning structural heart interventions, 22 out of 29 queries were chosen by the majority of participants.
Figure 2 shows the results of the group consensus. When the participants were encouraged to reach a consensus with other colleagues the number of queries deemed important for an EAPCI survey decreased from 15 to 11 concerning the demographic and organisational topic, and from 28 to 27 for the coronary intervention topic. In contrast, the number of queries increased from zero to three for the peripheral intervention topic, and from 22 to 26 for the structural heart disease topic.
During the brainstorming session there was consensus on not including queries on peripheral interventions in this early phase. Due to the large heterogeneity of professionals performing these procedures and of the pattern of data collection, there was concern about the reliability of the information generated.
Overall, the minimum number of queries deemed interesting/important for an EAPCI survey by the majority of participants (>50%) was, on average, 65 when chosen on an individual basis and 64 when chosen by group consensus (from a total of 115 proposed).
A final questionnaire was given to the participants in order to understand the individual needs and visions on constituting the survey better. In terms of the question as to whether they agreed on collecting aggregated data at country level (in the presence of a well-structured national registry) and at centre level by questionnaire (in the absence of a well-structured national registry), 88% responded positively, 7% were negative and 5% were uncertain. The main reason for the negative responses was the preference for a model of patient-level data collection. The uncertainty was motivated by the need to understand the clear objectives of the EAPCI survey and the return which national working groups would derive from participating in a common survey.
Of the working groups (WG) present, 76% declared having a national registry, mostly web-based, and the others, who had declared the absence of a national registry, disclosed the existence of local registries.
In most cases, either the president or the board of the WG is responsible for the national registry. In a few cases, data managers are responsible for the existing registries.
In most of the existing registries the percent of centres included was more than 80%, with local sites in charge of the collection and input of data into the registry. In a few cases, the local sites collected the data on paper report forms which were then sent to the national coordinator or data manager who is eventually responsible for inputting the data into the national registry.
Quality control on data collection and reporting is generally absent. There are, however, a few exceptions consisting either of a random audit on a small sample size (generally 10%) or, in a couple of cases, an independent and systematic audit of all the centres. In one case the data and the clinical outcome were evaluated by an independent clinical events committee.
Workshop 3. Challenges and solutions in building a common survey
The majority of participants agreed on adopting a mixed model consisting of: a) requesting aggregated country data from NCS or WG running well-structured registries; b) requesting (via annual questionnaires) aggregated centre-level data from the national WG representatives for the other countries.
There was a consensus that the above solution is provisional and dependent on the establishment of well-structured registries in all countries willing to upgrade and improve their national data collection. In particular, it was proposed that the EAPCI should support those countries to develop their own national registry, e.g., by providing a ready-to-use database (CARDS).
An annual EAPCI survey should have clear objectives. Those proposed were as follows: a) to provide reliable and regular feedback on the implementation of ESC/EAPCI guidelines to the scientific societies and to the writing committee; b) to support NCS or WGs with a recommendation letter from the EAPCI to the regulatory bodies of the respective countries in case of suboptimal guideline implementation; c) to boost the adoption of well-structured registries in those countries with suboptimal data collection; d) to provide a source for publication both at international and national level.
There was consensus on limiting the collection of clinical outcome data. In particular, if feasible, the collection of rough figures on in-hospital (in-centre) all-cause mortality is desirable.
There was consensus on requesting aggregated procedural data.
Possible financial means to support some countries in the collection of data was also advocated (e.g., EU funding).
Among the several ESC countries present at the Summit, the quality control of data collection is very limited and heterogeneous. There is an awareness that this remains a sensitive issue without financial means to support an independent audit system.
There was consensus that a clear agreement should be reached between EAPCI and the NCS or WG on the ownership of data. In particular, what the data will be used for and the role of the representative of the NCS or WG should be clarified.
Workshop 4. Deliverables of the survey
The results of the EAPCI survey thus constituted may be the object of multiple publication strategies. These data can be published in a time-sensitive fashion on the EAPCI website, or reported in newsletters, national or international journals (e.g., the European Heart Journal, EuroIntervention). The results can be communicated to both national or international meetings (EuroPCR, ESC, etc.).
Among the outcomes of the EAPCI summit, there will be a paper on the proceedings of the meeting. This will describe the general content and discussion points of the Summit and will be prepared as a short manuscript (500 words) to be published in EuroIntervention. An additional paper will be generated on the methodology to be followed for constituting the survey (project paper). In these two papers, the participants at the Summit should be acknowledged by inclusion in the authorship (either in the paper itself or on the online version).
The publication policy should be discussed openly and agreed upon at the time the working groups are asked to share their data. A possible model of publication policy for the main paper could be: a) inclusion of all of the presidents of the WGs participating in the survey, plus some EAPCI board members actively involved in the preparation of the manuscript; b) the president of EAPCI should be the senior author on the manuscript; c) in case of a limited number of authors per journal policy a rewarding criterion could consist of including those presidents (or data managers) who contributed the highest amounts of data (or including the participants as “contributors”); d) depending on the quality of the data, the main paper could be considered for the European Heart Journal.
From the main data set, additional papers could be prepared following novel sub-analysis to be considered for publication in EuroIntervention.
As a bonus additional manuscripts could be generated and published in a national journal and also in the local language. In these manuscripts, visibility can be assured by the national and local champions.
A potential special supplement of the EuroIntervention journal is being considered which would be dedicated to the publication of the registries of the national working groups. Further discussions are envisaged in the short term to develop this proposal.
Transparency should also be warranted in the policy of communication of the results to international and national meetings. Common slides could be shared among a selected group of presenters.
Industries are interested overall in a possible EAPCI survey. They are mostly interested in reimbursement policies and in other kinds of information of limited interest to the participants.

