Abstract
The value of myocardial perfusion scintigraphy (MPS) in predicting the occurrence of restenosis or new coronary lesions after stent implantation is debatable. A total of 47 patients treated with successful bare stent implantation underwent stress gated SPECT MPS at three time-points: pre-procedure, early pos-procedure, and 6-month follow-up. Follow-up angiographic re-study was obtained at 6 months. Overall, 51.1% of patients had angiographic in-stent restenosis or a new lesion at follow-up. Pre-procedure MPS and early MPS scans did not differ between patients with or without restenosis/new lesions. At follow-up transient perfusion defects were observed in 26.1% of patients without restenosis/new lesions and in 75.0% of patients presenting with restenosis/new lesions (p<0.01) (sensitivity: 75.0%; especificity: 73.9%). When comparing early post procedure MPS to follow-up MPS, patients without restenosis/new lesions had no changes or a decrease in the number of segments with transient defects (median difference 0 [interquartile range -2 – 0]), while patients with restenosis/new lesions had an increase in transient defects (+2 [interquartile range 2 – 3.75]; p<0.01). Two multivariate factors independently predicted new lesion/restenosis: reference diameter < 2.9 mm (OR 6.50; p=0.05) and the difference in the number of segments with transient defects between early post procedure and follow-up MPS (OR 1.87; p<0.01). In conclusion, pre-procedure and early MPS did not predict complications after coronary stenting, while follow-up MPS was suboptimal in differentiating patients with or without new lesions/restenosis. However, the change in myocardial perfusion from early post-procedure to the follow-up scan was an important prognostic factor to predict the incidence of new lesion/restenosis.
Introduction
Myocardial perfusion scintigraphy (MPS) with gated-SPECT is commonly utilised as a diagnostic tool for patients with suspected or already established coronary heart disease. Transient perfusion defects are commonly associated with the presence of flow-limiting coronary obstructions in these patients, who are frequently referred for percutaneous dilatation of the culprit stenoses. Interestingly however, MPS performed early after coronary intervention has been shown to have a poor correlation with angiographic findings at the end of the procedure1,2. In previous series, transient perfusion defects have been found in up to 77% of patients even after successfully accomplished percutaneous interventions3.
The clinical relevance of myocardial scintigraphy performed early after coronary intervention remains controversial4. In a previous study, the presence of transient perfusion defects detected in the first hours/days after a successful percutaneous intervention was associated with a high risk of late restenosis5. In addition, an early normal myocardial perfusion identified a group of patients with low restenosis rates at follow-up5. More recently, however, the need for target lesion revascularisation was not reliably predicted by early myocardial scintigraphy6. The pathophysiology of early transient perfusion defects after percutaneous interventions is currently unclear, with local spasm and recoil at the treated site, release of vasoactive agents, and unnoticed residual stenosis being previously postulated as possible mechanisms7-9. Interestingly, although coronary stenting potentially minimise many of these theoretical factors, no clear difference has been found in early MPS between patients treated with balloon or stent6. The present study aimed to assess the prognostic value of sequential scintigraphic myocardial perfusion scan performed at baseline, early after stenting, and at follow-up. The ability of myocardial scintigraphy to predict the clinical outcomes and the incidence of restenosis or a new lesion during follow-up was evaluated in a series of cases with coronary disease treated with contemporary percutaneous techniques.
Methods
Patient population and study design
Inclusion criteria included stable coronary disease, successful coronary bare stent implantation (residual diameter stenosis < 30% without major in-hospital complications), target lesion with pre-procedure diameter stenosis > 70% (by visual analysis), and pre-procedure transient myocardial perfusion abnormalities in the territory of the stented artery. Gated SPECT myocardial perfusion scintigraphy studies were performed within 48 hours after the procedure (early MPS) and was repeated at 3 to 6 months thereafter (follow-up MPS). Also, a coronary angiogram was performed 3 to 6 months post-stenting (follow-up angiogram). Patients were clinically evaluated before and at 30, 60, 90, and 180 days after coronary stenting.
From a total of 50 patients initially screened, 2 patients refused to undergo the follow-up angiographic re-study and were not included in the present report. An additional patient was excluded because of stent thrombosis needing urgent re-intervention (with no preceding MPS) two months after the intervention. Thus, the present study population comprised 47 patients with complete sequential scintigraphic and angiographic evaluation. This protocol was approved by the local ethics committee and is in accordance with the Declaration of Helsinki. Written, informed consent was obtained from every patient.
Coronary angiography and percutaneous interventions
Coronary lesions were classified according to Ellis et al10. Quantitative coronary angiographic analysis was performed as previously described, utilising a validated computer-based edge-detection system (CASS II, Pie Medical, Maastricht, The Netherlands) with the catheter tip as the calibration factor11. Binary angiographic restenosis was defined as a diameter stenosis > 50% at the follow-up angiography. Late luminal loss was calculated as the difference between the minimal luminal diameter immediately after stenting and at follow-up.
All percutaneous interventions were performed with stent implantation according to standard procedures with the final strategy left at the discretion of the operator. Patients were maintaned on lifelong aspirin and clopidogrel 75 mg qd for a month.
Myocardial perfusion scintigraphy
Myocardial perfusion scintigraphy was performed using a one day protocol with rest and stress, according to the routine of our institution. Initially, all patients received at rest an intravenous dose of 370 MBq 99mTc sestamibi. Images were acquired every 2.8° using a gamma-scintillation camera (Sopha Medical model DST coupled with two Sophy-NXT computers and one Vision Power Station) with a high-resolution collimator and a circular orbit (180°) starting at a 45° right anterior oblique position. Reconstructed images were obtained in short-axis, horizontal long axis, and vertical long axis sections (Butterworth filter; order 5; section frequency 0.18).
The stress phase scintigraphy was acquired after physical exercise or pharmacological stress. For an individual patient, the same stress strategy (either exercise or pharmacological) was maintained for every MPS examination (16 patients had exercise stress testing and 31 had pharmacological stress [26 by adenosine and 5 by dipyridamole]). Exercise tests were performed using the Bruce protocol12 and were interpreted according to ACC/ AHA guidelines13. At peak exercise an additional intravenous dose of 1110 MBq 99mTc-MIBI was injected. Images were acquired approximately 30 minutes after administration of the radiopharmaceutical. Patients undergoing pharmacological stress received intravenous dipyridamole (0.56 mg/kg of body weight during 4 minutes) or adenosine (140 microgram/kg of body weigh per minute for 6 min). Three minutes after dipyridamole or adenosine infusions, another intravenous dose of 1110 MBq 99mTc-MIBI was injected. Images were acquired 60 to 90 minutes later and reconstructed in the same way as rest images.
The stress images were gated to the patient’s ECG. Stress gated SPECT study was divided in eight segments, regional and global wall motion and left ventricular ejection fraction were evaluated using a commercial program13.
Scans were divided into 17 myocardial segments, corresponding to the location of coronary artery territories14. Perfusion characteristics were assessed by two experienced observers blinded to the clinical and angiographic data. A semi-quantitative graded score was used:
Grade 0: normal perfusion;
Grade 1: mildly reduced perfusion;
Grade 2: moderately reduced perfusion;
Grade 3: severely reduced perfusion;
Grade 4: absence of perfusion.
The diagnosis of reversible hypoperfusion was made by a perfusion grade 2 to 4 present on stress images in at least 2 axes or in 2 consecutives tomographic sections on the same axis, with improvement to grade 1 or 2 at rest.
Statistical analysis
Categorical variables are expressed as frequencies. Continuous variables are expressed as mean±SD. Statistical analysis of the number of segments with various degrees of abnormal perfusion before, early and 3 to 6 months after intervention was performed with reference to all the patients and to the subgroups of patients with and without restenosis / new lesions using repeated measures analysis of variance.
Univariate analysis was performed using the Fischer exact test for categoric values and the nonparametric Mann-Withney test for continuous variables. A p value < 0.05 was considered statistically significant. A multivariate logistic regression analysis was performed to evaluate the value of myocardial perfusion scintigraphy to predict in-stent restenosis or the appearance of a new lesion during follow-up. Well-known risk factors for restenosis (diabetes, reference vessel diameter, lesion length, and post-procedure minimal luminal diameter) were tested together with variables derived from the scintigraphic findings. The lack of fit and the predictive ability of the best selected model was evaluated by the Hosmer and Lemeshow Test and the C-index, respectively.
Results
From the 47 patients included, 30 were men (64%) and the mean age was 59±9 years (Table 1).
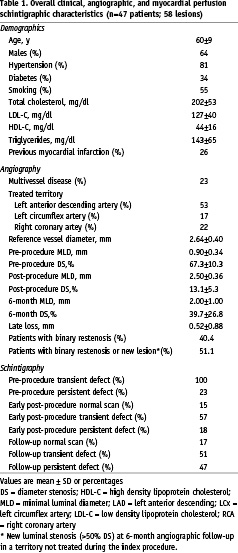
Diabetes was present in 18 patients (38%) and 28 patients (60%) were smokers. Thirty seven patients (79%) had angina before the intervention (Canadian Cardiovascular Association class (CCS) I: 26%, CCS II: 70%, CCS III: 4%) and 13 patients were asymptomatic. A total of 15 patients (32%) had multivessel disease. Complete revascularisation (absence of residual stenosis > 50% in any coronary vessel) was achieved in all patients with single vessel disease and 4 patients with multivessel disease (in total 36 patients [77%] had complete revascularisation). In 11 patients (23%), other lesions with diameter stenosis > 50% were left untreated due to the absence of scintigraphic ischaemia in the corresponding myocardial segments.
Angiographic analysis and clinical follow-up
In total, 58 lesions were stented (1 lesion was stented in 36 patients [77%] and 2 lesions were treated in 11 patients [23%]). The LAD was dilated in 23 patients, the RCA in 7 patients, and the LCX in 6 patients. In the 11 cases of 2 angioplasties performed in the same patient, the LAD and the LCX were treated in 3 patients, the LAD and the RCA in 4 patients, the RCA in more than one site in 2 patients, the LAD in more than one site in 1 patient and the LCX in more than one site in 1 patient. Angiographic findings are presented in Table 1. The left anterior descending artery was treated in approximately half of cases. Mean reference diameter was 2.65±0.41 mm and the average residual stenosis after stenting was 13.0±0.1%. The follow-up coronary angiography was performed 5.5±1.2 months after the procedure. Binary restenosis was observed in 19 of the dilated lesions (32.8% of the 58 stented lesions and 40.4% of the 47 patients who completed the study). In addition, 5 patients presented new significant obstructions in a non-treated vessel at the follow-up angiography. Therefore, a total of 24 patients (51.1%) presented in-stent restenosis or a new lesion during follow-up. The average late luminal loss at the treated segment was 0.52±0.88 mm.
There were no deaths during follow-up (Table 2).
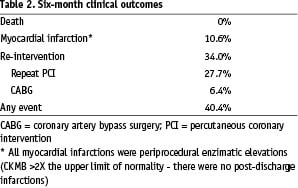
The incidence of periprocedural myocardial infarction (CKMB elevation > 2X normal level) was 10.6% (all non-Q wave myocardial infarction) and there were no other myocardial infarctions during follow-up. In total, 16 patients (34.0%) underwent repeat interventions during follow-up (repeat angioplasty: 27.7%; CABG: 6.4%).
Myocardial perfusion scintigraphy findings
Baseline myocardial perfusion scintigraphy scans were obtained 7.2±10.9 days before the angioplasty and all post-stenting MPSs were obtained 24 to 48 hours early after the procedure. Follow-up scintigraphies were acquired on average after 5.4±1.1 months (8.9±9.0 days before the follow-up angiography).
Pre-procedure MPS scans showed at least one segment with transient perfusion defects in all cases and persistent residual perfusion abnormalities in 11 patients (23%) (Table 1). Early post-procedure MPS was normal in 7 patients (15%), had reversible myocardial perfusion defects in 27 patients (57%), and areas of persistent perfusion abnormalities in 18 patients (38%) (Table 1). When compared to the pre-procedure MPS, 41 patients (87%) had a decrease in the number of transient perfusion defects in early MPS, while the remaining cases were unchanged (3 patients [6%]) or had an increase in the number of segments with transient defects (3 patients [6%]). At follow-up, 8 patients (17%) had normal scans, 24 patients (51%) had reversible defects, and 22 patients (47%) had irreversible perfusion defects (Table 1).
The association between MPS findings and the incidence restenosis or the appearance of new lesions is shown in Table 3.
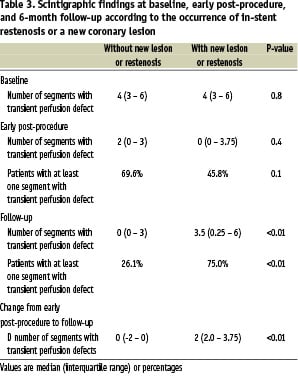
Pre-procedure MPS and early MPS scans did not differ between patients with or without restenosis/new lesions. Conversely, follow-up MPSs were significantly different between the two groups. Transient perfusion defects at follow-up were observed in 26.1% of patients without restenosis/new lesions, while 75.0% of patients presenting with restenosis/new lesions had at least one segment with transient perfusion defect at follow-up (p<0.01). The presence of at least one segment with transient perfusion defect at follow-up had a sensitivity and especificity to detect restenosis/new lesions of 75.0% (95% CI: 53.3% – 90.2%) and 73.9% (95% CI: 51.6% – 89.8%) respectively. Moreover, the change in the number of segments with transient defects was significantly different between the groups (Table 3). Patients without restenosis/new lesions remained stable or had a decrease in the number of segments with transients defects between post-stenting and follow-up (median difference 0 [interquartile range -2 – 0]), whereas patients with restenosis/new lesions had an increase in the number of segments with transient defects between post-stenting and follow-up (2 [interquartile range 2 - 3.75]; p<0.01) (Table 3).
Figure 1 shows the relationship between MPS findings and restenosis/ new lesions.
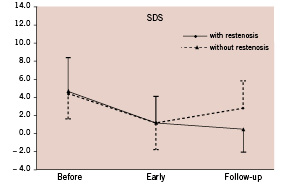
Figure 1. Comparative analysis of Summed Difference Score in myocardial perfusion scintigraphy.
Pre-procedure and early post-stent MPS were not predictive of any clinical or angiographic event. However, at follow-up, the number of segments with transient defects was significantly associated with new lesion/restenosis (OR 1.44; 95% CI: 1.12 – 1.87; p<0.01), repeat revascularisation (OR 1.32; 95% CI: 1.05 – 1.68; p=0.02), and total event (OR 1.28; 95% CI: 1.02 – 1.61; p=0.03). Interestingly, the change (difference) in the number of segments with transient defects between early and follow-up MPS was also significantly associated with the risk of new lesion/restenosis (OR 1.77; 95% CI: 1.22 – 2.57; p<0.01), repeat revascularisation (OR 1.46; 95% CI: 1.09 – 1.95; p=0.01), and total event (OR 1.40; 95% CI: 1.06 – 1.85; p=0.02).
Two multivariate factors were identified to independently predict new lesion/restenosis: reference vessel diameter < 2.9 mm (highest quartile in our series) (OR 6.50 [95% CI: 1.00 – 42.28]; p=0.05) and the difference in the number of segments with transient defects between early and follow-up MPS (OR 1.87 [95% CI: 1.24 – 2.83]; p<0.01) (final model constant: -1.63; Hosmer and Lemeshow statistic: 3.9 [p = 0.7]; C-index: 0.86). Similar findings were observed when the risk of restenotic lesions was analysed isolatedly (without the occurrence of new angiographic lesion at follow-up).
Mean ejection fraction pre, early, 3 months and 6 months after angioplasty were 47.8% (SD 9.5%, confidence interval 2.6%), 48.0% (SD 8,6%, 95% confidence interval 2.4%), 50,6% (SD 7.4%, 95% confidence interval 2.0%) and 50,4% (SD 9.4%, 95% confidence interval 2.6%), respectively (p=NS for all).
Discussion
We found that scintigraphic myocardial perfusion imaging performed early after succesful coronary stenting was frequently abnormal with approximately 60% of patients still presenting transient perfusion defects. Moreover, early MPS did not predict the incidence of future restenosis or new coronary lesions. Also, myocardial scintigraphy performed at 6-month follow-up had only a suboptimal ability to differentiate patients with or without new lesions/restenosis (sensibility and especificity of only ~75%). Interestingly however, the change in the number of segments with transient perfusion defects between early and follow-up MPS was identified as an important predictor of the incidence of new lesions/restenosis at follow-up. The detection of one extra segment with transient perfusion at follow-up, compared to the post-procedure scan, increased the risk of new lesion/ restenosis by 87%. Conversely, improvement of MPS at follow-up, when compared to early MPS, almost halved the chance of these events.
Identification of a significant coronary artery lesion later after revascularisation was only 75% in this study because we used binary restenosis considering 50% of lumen reduction. This angiographic criteria was used because it is important to compare scientific protocols, this may induce great difference in treatment of restenosis between angiographic stenosis and guided medical indication. When we compare myocardial perfusion scintigraphy performed 48 hs after the procedure with the subsequent scan we had worsening of myocardial perfusion in 16 of 24 patients. Eight patients that did not show worst perfusion pattern when compare with early image showed new lesions (false negative), 7 had in stent restenosis with lesions ranging between 51 to 60%. Another patient showed a new lesion in another vessel, with 84% of stenosis with mild collateral to your distal branch. In summary, 7 among 8 patients with false negative studies had stent restenosis with luminal diameter not superior to 60%. Eight out of 18 patients that had in stent restenosis did not complain of angina in the follow-up, only one of these patients referred chest pain during stress test, but all had worst follow-up scan imaging when we compared to early image. An interesting issue was that 6 among 8 patients referred angina before the procedure, and only one among these 6 had a small increase of collateral circulation in comparison to pre-procedure angiogram.
There were no significant improvement in LV global and/or regional function 6 months after the procedure. Visual analysis of the gated SPECT images showed that in only one patient the motility worsened early after the procedure, probably due to myocardial stunning. It seemed that motility data did not have incremental value over the perfusion studies in this analysis.
Similar to our findings, Jaffe et al found that early 201-thallium scintigraphy did not predict late target lesion revascularisation6. Conversely, Kostkiewicz et al showed that normalisation of myocardial perfusion in MPS obtained early after plain balloon angioplasty was associated with a significantly lower rate of restenosis when compared to patients with areas of residual post dilatation myocardial hypoperfusion3. Curiously, similarly to our findings, the recurrence of stenosis was frequently associated with a late increase in the number of segments with transient ischaemia in comparison with scans obtained 6-10 days after the procedure3. The absence of ischaemia on thallium SPECT imaging at 5 months after coronary stenting has been previously shown to indicate a low risk for cardiovascular events or interventional procedure15.
The possible mechanisms for early false positive scans following coronary interventions remain unproven. Since stents inhibit local spasms and elastic recoil, it seems unlikely that residual stenosis/spasm in the epicardial vessels may contribute to these findings. Microvascular transient functional abnormalities due to microembolisation, localized stunning, and/or liberation of vasoactive substances might contribute to the temporary perfusion abnormalities. However, no relationship was observed in our cases between post-procedure myocardial infarction (CKMB release) and the presence of early transient defects.
Our data suggest that a scintigraphic scan performed within 48 hours after stenting may add to the ability in predicting new/lesion restenosis at follow-up. The comparison between early post-procedure and the follow-up scan yielded the most important prognostic factor to predict the incidence of new/lesion restenosis in our study. This way, early MPS would function as a ‘fingerprint’ imaging after successful stent implantation, against which later scans could be compared. During follow-up, worsening of the scintigraphic image was indicative of either restenosis or the development of new obstructive coronary lesions. Conversely, the improvement of scintigraphic findings during follow-up indicated a low risk for the occurrence of these complications.
Identification of a significant coronary artery lesion later after revascularisation was only 75% in this study because we used binary restenosis considering 50% of lumen reduction. This angiographic criteria was used because it is important to compare scientific protocols; this criteria may induce great differences in indices of reinterventions when comparing angiographic and guided medical indications. When myocardial perfusion scintigraphy performed 48 hours after the procedure was compared to the subsequent scan there was worsening of myocardial perfusion in 16 of 24 patients. Eight among these 16 patients showed new lesions (false negative results). However, in 7 the in stent restenosis ranged from 51 to 60%. One patient developed a new severely obstructive lesion in a vessel not treated in the index procedure. In summary, 7 among 8 patients with false negative studies had stent restenosis with luminal diameter reductions not superior to 60%. Eight out of 18 patients that had in stent restenosis did not complain of angina in the follow-up; only one of these patients referred chest pain during stress test, but all had worse follow-up scan imaging when compared to early images.
There were no significant improvements in LV global and/or regional function 6 months after the procedure. Visual analysis of the gated SPECT images showed that in only one patient the motility worsened early after the procedure, probably due to myocardial stunning. It seemed that motility data did not have incremental value over the perfusion studies in this analysis.
Study limitations
Scintigraphic images in this study were analysed by semiquantitative evaluation. However, in a large series of patients, expert visual analysis was found to be similar to automatic quantitative analysis of myocardial perfusion SPECT for prognostic information16. Our purpose was to determine the effectiveness of the most commonly applied clinical approach, which is visual interpretation. Moreover, the relative diversity in the techniques applied (either exercise or pharmacological stress with dipiridamol or adenosine) reflect a more realistic observation of the common clinical practice of a high volume interventional cardiology laboratory and, consequently, may be more easily applicable to the general population. Parallel application of exercise and pharmacological stress to evaluate myocardial perfusion reserve is widely accepted. For patients who cannot exercise, pharmacological stress provides more reliable test results than ineffective exercise17,18. It is important to emphasise that, for an individual patient, the same protocol was used for all sequential MPSs. We did not have any side effects after all stress tests, even performed early after the procedure, and we did not change any medications during the protocol.
Conclusions
Early post-procedure or late follow up myocardial perfusion scintigraphy were not able in isolation to predict future complications after coronary stenting. However, the change in myocardial perfusion between early post-procedure and follow-up scans yielded the most important prognostic factor to predict the incidence of restenosis/new lesion in our study. The present findings suggest that the acquisition of an early MPS for comparison with late sequential scans may represent a valuable clinical tool for the detection of restenosis or new coronary lesions after bare metal stenting and possibly might be useful for patients in whom a pre procedural scan was not available. While drug eluting stents (DES) have reduced the restenosis rates, there are still many places where DES are used in a minority of patients. Our data is particularly applicable to patients treated with one or more bare metal stents where restenosis is still problematic.