Abstract
As a result of the introduction of drug eluting stents (DES) to clinical practice, angiographic and clinical parameters of restenosis have been significantly improved. However, several recent publications have raised concerns about long-term safety of this technology. They include a potential risk of inducing chronic inflammation within the coronary artery, delayed healing and late stent thrombosis.
Recently, late stent thrombosis, a rare but often life threatening event, has been reported to occur more frequently following DES placement. The mechanism of this phenomenon has not been fully elucidated.
What is the true incidence of stent thrombosis after DES therapy? Is it similar or higher than with bare metal stents? Are randomised trials with DES therapy overestimating the benefits of this therapy? Which are the potential limitations of these studies? Are DES increasing rates of death and myocardial infarction from randomised trials and registries a true fact? In the following pages we review recently reported data about DES suggesting potential safety concerns associated with the current widespread use of DES.
Introduction
Since the introduction of drug eluting stents (DES) for percutaneous coronary interventional procedures, angiographic and clinical parameters of coronary restenosis have strikingly decreased during the first years of follow-up. Several randomised studies comparing bare metal stents (BMS) versus FDA approved DES designs, sirolimus eluting stent (SES) and paclitaxel eluting stent (PES) in patients with low-risk coronary lesions demonstrated a significant reduction in coronary restenosis which translated into lower target vessel revascularisation (TVR) and target lesion revascularisation (TLR) rates1-5. Following approval, we have witnessed a widespread use of DES technology to include higher-risk patients and complex lesions such as overlapping stents for long lesions, bifurcating lesions and left main stenosis, even in patients with non-severe obstructive coronary disease6-8.
However, after four years of carrying out an almost unselected, systematic use of these devices in the United States and in many countries throughout the world, the angiographic and clinical improvement of the rate of restenosis has not been translated into a reduction of the two strongest and most powerful cardiac events i.e., myocardial infarction or death.
Moreover, recently worrisome data showed a significant increase of such severe events with the use of DES in predetermined clinical circumstances, and/or lesion or procedural characteristics6,7.
The “problem” of coronary restenosis
Following the introduction of percutaneous coronary balloon angioplasty, coronary restenosis was identified as the Achilles Heel of this technique. Elastic recoil, negative arterial wall remodelling and neointimal hyperplasia were identified as the underlying mechanism of this phenomenon. After the introduction of coronary stents9-11, neointimal hyperplasia remained the sole factor associated with coronary restenosis after BMS implantation. Therefore, its prevention should be related to therapies that inhibit smooth muscle cell proliferation1-5,12,13.
Through the years, we have also learned that angiographic coronary restenosis after percutaneous coronary interventions (PCI) is a prevalent, but also a “soft” and clinically irrelevant event. This concept is supported by the results of several randomised studies comparing a variety of percutaneous approaches that have shown a significant reduction in angiographic and clinical parameters of restenosis after implantation. These studies consistently failed to show any reduction in cardiac mortality and/or myocardial infarction (Figure 1). In randomised studies comparing BMS vs. plain optimal balloon angioplasty (POBA), provisional vs. universal stenting9,10,14,15-17, POBA vs. coronary bypass surgery (CABG), BMS vs. CABG or lately BMS vs. DES18-23; restenosis and TVR reduction are not associated with similar reductions in death and/or myocardial infarction. Even though, from a patient’s point of view, restenosis is not an irrelevant event, we also know that since the dawn of PCI, coronary restenosis is a non life-threatening event... otherwise, it would have been impossible for percutaneous interventions to be a feasible alternative to CABG.
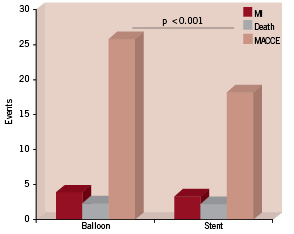
Figure 1. Meta-analysis from 25 randomised trials comparing a strategy of routine stenting with that of balloon angioplasty plus provisional stenting. No difference in death and myocardial infarction with significant decrease in MACCE due to lower restenosis. (Adapted from Al Suwadi, et al. Am Heart J 2004;147:815-22).
Moreover, excluding the diabetic population, all individual randomised studies have shown no significant differences at mid-term follow-up in death and/or non-fatal myocardial infarction between CABG and POBA or BMS18,19. In addition, the long-term follow-up of the SIRIUS and TAXUS trials once again demonstrated that restenosis is a benign event. Pooled analysis from these randomised studies over four years reported an extremely lower restenosis rate with DES as compared to BMS. However, this lower restenosis rate failed to translate into any advantage in the incidence of death and/or non-fatal myocardial infarction24,25.
If we take into account the aforementioned findings as we search for therapies with the potential to reduce restenosis, we cannot accept any increase (not even a few) in “hard” cardiac events (death and myocardial infarction) as a price to pay to achieve such a target.
The DES Revolution
Following the introduction of DES in clinical practice at the beginning of this century, the use of both SES (Cypher™, Cordis Corp., Miami Lakes, Florida, USA) and PES (Taxus™, Boston Scientific Corp., Natick, Massachusetts, USA) have provided a significant reduction of angiographic restenosis, demonstrating that the stent polymer coating is a good platform for storing the drug and defining a release mechanism. However, several recent publications have raised concerns about long-term safety issues, such as the potential risk of inducing chronic inflammation within the coronary artery, delayed healing and late stent thrombosis26-28.
Stent thrombosis with BMS was observed during the early years following the introduction of this therapy29, however, after the introduction of thyopiridines (ticlopidine and clopidogrel) frequency of this event became very rare and was not a major concern with the use of BMS30,31. Later on, late stent thrombosis became a major concern following coronary brachytherapy32.
The issue of late stent thrombosis with DES is a contentious one. The pathophysiology of this severe event could be linked to the polymer and/or delayed re-endothelialisation caused by the long-term drug release, or to a toxic local effect during drug release over the endothelium.
What is the true incidence of stent thrombosis after DES therapy? Is it similar or higher than BMS? Are randomised trials with DES therapy overestimating the benefits of this therapy? What are the potential limitations of these studies? The problem of stent thrombosis after DES deployment can briefly be characterised as a not so frequent, but worrisome cardiac event.
What does that mean?
Sudden cardiac death and acute myocardial infarction have been frequently associated with stent thrombosis regardless of the chronology of the event (sub acute, late and very late). If patients are protected by the clopidogrel “umbrella”, stent thrombosis rates would probably not differ between DES or BMS. However, recent data clearly demonstrates that clopidogrel cessation in DES patients results in a significant increase in the risk of stent thrombosis. Although several independent factors have been associated with this finding, clopidogrel discontinuation, either early or late, has been identified as the most powerful predictor of this event after hospital discharge8,33-37.
The disturbing observation that stent thrombosis could appear late and very late – beyond one, two or more years after stent deployment – means that dual antiplatelet therapy should be taken for one or more years, perhaps indefinitely. Recent data from the Basket-Late Trial and from the Duke Registry, stressed the role of clopidogrel discontinuation after DES implantation. Both studies showed significantly higher incidence of death and MI in patients where clopidogrel was discontinued. Thus, extended use of clopidogrel in patients with DES is associated with a reduction in the risk of death and myocardial infarction at 6, 12 and 24 months of follow-up36,37.
However, if late stent thrombosis is not so frequent, should we be concerned? The answer is yes, we should. If an infrequent, but severe clinical event is difficult to identify, it could be potentially more severe than if it was frequent. An example of this is the earlier experience with the 7-hexanoyltaxol-eluting stent38, which was one of the first eluting stent designs. The use of this stent was associated with an extremely high incidence of stent thrombosis, which was associated as well with a high incidence of death and myocardial infarction. These findings determined the premature cessation of the randomised trial and the removal of such stent design from clinical practice. This is a good example illustrating that when a severe event is frequent and easy to identify, it can be paradoxically not so severe. If sudden death or myocardial infarction is frequently associated with this event, can an increase in late or very late stent thrombosis be translated into high mortality? If we look at the pooled data of SIRIUS (RAVEL, SIRIUS,C-SIRIUS and E-SIRIUS) trials at four years of follow-up24, the group of patients treated with SES had a trend to higher mortality (1.4% more) than patients treated with BMS. Furthermore, patients treated with BMS had significant higher numbers of diabetics (p=0.026) than patients treated with SES. It is well known that the presence of diabetes is an independent predictor of high mortality after PCI procedures39. Thus, we can speculate that if the control BMS group has more diabetics, this patient population could have an inferior survival rate. In other words, in the pooled analysis of the SIRIUS trials, the population with higher cardiac risk baseline characteristics (BMS) had a better survival at four years of follow-up than the SES population, stressing worst survival with DES. The five year follow-up of the RAVEL trial reported a higher rate of death and myocardial infarction with SES40, and a recent meta-analysis from 17 randomised DES trials41 also demonstrated extremely disturbing data showing a poor outcome with DES therapy (Figure 2). The findings in the SES treated patients of less incidence of diabetes and worse survival, underline the fact that coronary restenosis (which occurred more frequently in the BMS group) is a mild and non life-threatening event. In addition, from a scientific standpoint, it is not acceptable to define non-cardiac death without pathological examination, especially when we are testing an almost lifelong drug release device such SES or PES in which it is unclear when the drug is totally eluted. Furthermore, it is well known that cancer, which was the main cause of non-cardiac death in the RAVEL trial, is frequently associated with thrombosis and an hypercoagulability state. In addition, the majority of patients with cancer most likely had stopped clopidogrel during their treatment. In our stent thrombosis database34,43, three patients had their clopidogrel discontinued for cancer treatment and developed an acute coronary syndrome and myocardial infarction during hospitalisation. If these patients would have been treated in a hospital without cardiac catheterisation facilities and died, how should their death been classified? Would these patients have been classified as having died from stent thrombosis or be placed within the category of non-cardiac deaths?
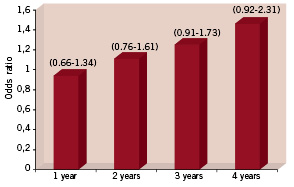
Figure 2. Odds ratio for overall mortality in patients with DES implantation vs. patients with bare metal stent implantation in randomised trials. Adapted from Nordmann AJ, Briel M, Bucher HC. Mortality in randomised controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006.
Finally, we should not discard, as shown by angioscopic and intravascular ultrasound images studies, that some focal restenosis, the most frequent angiographic finding of sirolimus stent restenosis, could represent indeed stent thrombosis as the underlying mechanism involved in such event28,42.
In the ERACI III, a multicentre prospective registry conducted with the purpose of comparing the outcome of DES with a similar patient population treated either with BMS or coronary bypass surgery, there was reported a trend to a higher incidence of stent thrombosis with DES during the first year: 2.6% vs. 1.3% for DES and BMS respectively. Furthermore, these differences increased over time to 3.5% vs. 1.3% and 4.4% vs. 1.3% at the end of the second and third year of follow-up respectively (p=0.08), meaning a 0.9% increase rate per year. On the contrary, beyond 30 days of the initial procedure, no patient treated with BMS suffered late or very late stent thrombosis, whereas 9 patients developed that complication after DES placement (p=0.008). Of further interest, those patients having concomitant DES and BMS placement in different arteries and with angiographic documented stent thrombosis, all of the thrombosis occurred at the DES sites, strongly implying a device specific aetiology in late stent thrombosis. Interestingly, at the three year follow-up, diabetic patients treated with DES in the ERACI III, had a significantly higher incidence of death (p=0.032), death plus non-fatal myocardial infarction (p=0.031) and MACCE (p=0.018), as compared to non-diabetics, demonstrating a loss of maintenance in the initial benefit observed during the first year of follow-up23,34. Furthermore, and in agreement with this finding, a recent report from the ThoraxCentre in diabetics43 also observed a loss of initial benefit with SES over BMS at the second year of follow-up, with higher incidence of late stent thrombosis with SES compared to those treated with BMS (4.4% vs. 0.8%, p=0.015). A large French registry44 recently reported that insulin dependent diabetics treated with SES had a stunning 6.0% rate of stent thrombosis, which translated into higher incidence of death and myocardial infarction.
All of these recent findings convey the questionability of the alleged superiority of DES compared to BMS in diabetics. However, these results should be taken with caution considering the non-randomised nature of these studies and the small sample size patient population.
Pre-clinical data with these devices
At the beginning, DES was launched into the market without extensive and solid basic animal data supporting the benefits of DES.
If we search medline for SES, sirolimus and/or rapamycin, the most frequent animal data reported was with the systemic use of the drug. Only a few studies were found with sirolimus loaded coated stents in the animal model. Furthermore, there was no published analysis on the long-term effects of SES in animal models before clinical studies were initiated45,46. In the first clinical observational study, a slow (>28 days drug release) and fast (<15 days drug release) formulation of the drug were used at a concentration of 140 micrograms per cm2 on stent struts1. In the commercially available SES, only the slow release of the drug is available with a similar concentration of drug in the stent struts. That means a concentration at the angioplasty site of 71,000 to 314,000 ng/cm2 of the sirolimus, depending on stent size. With this formulation, all randomised studies and registries found a significant decrease in late loss and restenosis with non-reported safety concerns such as stent thrombosis, cardiac death or non-fatal myocardial infarction. However, the high doses of the drug within stent struts could have potentially developed an excessive and prolonged vessel injury and thus be responsible for the worrisome findings associated with DES therapy in the long term follow-up. These findings include: delayed healing, lack of endothelialisation, stent late malapposition and/or partial or complete stent thrombosis. Late stent malapposition, a potential factor associated with stent thrombosis, has been described in 14.5 percent and 8 percent with SES and PES respectively47.
If we compare the local eluting approach with the systemic use of sirolimus, our concerns increase further. Oral administration of rapamycin after BMS implantation during 14 days achieved 13 to 14 ng/ml of sirolimus in peripheral blood samples12,13, and this concentration is enough to obtain a 40% reduction in late loss. On the contrary, the local administration, 300,000 ng of sirolimus per cm2 of stents struts seems to be excessive and beyond the safety/therapeutic level of sirolimus to the endothelium site.
Lessons learned from DES randomised studies
Have we overestimated the benefits of DES in randomised trials?
The answer is probably yes, we have. Studies with DES therapy have three major areas of concern:
– First, the relative lack of a biological background, with very few animal studies conducted with the use of rapamycin or paclitaxel eluting stents. If previous biological data opposed the results of phase 2 or 3 studies, the clinical implications of those trials should have been taken with more caution.
– Second, we shouldn’t have followed the path of trials using surrogates, only irrelevant, clinical endpoints such as angiographic or clinical parameters of restenosis in well-selected populations, with a relatively short-term follow-up. In medicine, any established therapeutic approach should always require long-term outcome analysis using hard clinical end points. In contrast, in recent years, several DES studies have been reported using the same clinical and angiographic soft end points at a short-term follow-up period.
– Third, it is unclear that DES therapy is not cost-effective in the major proportion of lesion subsets. When we analyse appropriately the SIRIUS trials3,48,49, the rate of angiographic restenosis in control arms have an extremely high incidence of restenosis (over 40%) using thick struts stent design. These numbers are far from the last generation chromo-cobalt thin struts stent design used in the BASKET trial. A cost effectiveness analysis recently reported by this study only justified the use of DES in selected high-risk groups50. At the present time, it appears that small vessel size and in-stent restenosis are the sole primary cost effective indication for DES therapy.
Finally, in closing, our request to the interventional cardiology community... It is the perfect time to refocus and redefine the true role of DES in clinical practice. Our goal is to provide our patients with a safe and dependable PCI procedure and we cannot settle for a procedure that would reduce a rather benign restenosis process, but could be associated with increased rates of stent thrombosis, unpredicted late mortality and non-fatal MI.

