Intracoronary thermography is a potential vulnerable plaque detection technique of which the development is ongoing. This review discusses the past and present status of this technique from a more fundamental perspective, in which heat generation, heat transfer and heat detection are considered. Details of the presumed heat source, the macrophage clusters in vulnerable, atherosclerotic plaques, are discussed, including their increased energy consumption and heat production values. Heat transfer by ways of conduction, convection by blood flow and radiation are considered in a quantitative way. Ways to detect heat that are currently used are discussed and suggestions for improvement as well as for utilization of unexplored possibilities are given. This review enables interpretation of previously published results and likely also of results to be published in the future.
Introduction
Heart disease is the number one cause of death in the Western world and number two in the non-Western world1. Coronary atherosclerosis is by far the most frequent cause of ischemic heart disease2, and it can be subdivided into a number of developmental stages. One of these stages involves the vulnerable plaque.
Plaque disruption with superimposed thrombosis is the main cause of events such as the acute coronary syndromes of unstable angina, myocardial infarction, and sudden death2. Three types of plaques can be identified as causes of thrombosis: the thin-cap fibroatheroma (TCFA); the plaque prone to erosion; and the plaque with calcified nodules. Of these the TCFA is best described as a “suspected” high risk/vulnerable plaque, since, while confirmatory data is lacking, its structure resembles that of ruptured plaques3. The major components of a TCFA are a lipid-rich, atheromatous core, and a thin fibrous cap with macrophage and lymphocyte infiltration together with decreased smooth muscle cell content3.
It is suggested that increased plaque heat is a feature of plaques that are denuded and inflamed and consequently at risk of thrombosis4. This heat might be due to increased metabolic activity of inflammatory cells, such as macrophages, or due to enzymatic extracellular matrix breakdown. Detection of heat could thus possibly lead to detection of vulnerable plaques.
The use of vulnerable plaque detection has recently been discussed5. The benefit of locating individual vulnerable plaques in a multifocal disease is a topic of debate. Detecting individual vulnerable plaques can be valuable for a number of reasons. Firstly, the natural history of the vulnerable plaque is not fully known and to obtain more information on the development of a vulnerable plaque one needs to be able to locate the individual vulnerable plaques. Secondly, pharmacological treatment of vulnerable plaque needs to be verified. And thirdly, there will be a group of people, of which the number is unknown, that will rely on invasive treatment due to failure of pharmacological treatment for whatever reason. In addition, effects of pharmacological agents can take a while to become effective, in which period it might be necessary to detect the individual plaques, perhaps to even treat them invasively. For these reasons one needs vulnerable plaque detection techniques that are well validated.
After the first publication in this relatively new field in cardiology, the detection of vulnerable plaques using thermographic methods4, many thoughts and results on this detection technique have been published. In figure 1 some of the published temperatures have been plotted versus time. The temperature differences that were published have decreased over time, but are still of significant value to conclude that thermal heterogeneity exists in vivo. Based on these values it is worthwhile to continue studying the exact details of the origin and the consequences of this thermal heterogeneity. This review will try to determine what needs to be measured and which pitfalls arise when measuring temperatures in vivo. In addition, it suggests ways to overcome these problems.
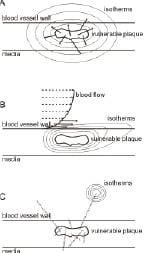
Fig. 1: A selection of published intracoronary thermography data. Temperatures measured either ex vivo on human atherosclerotic tissue (a) or in vivo in human coronary arteries (b through m).
Solid lines: reported temperature ranges, symbols: reported mean values
Diamonds: needle thermistor, squares: hydrofoil thermistor catheters, downward triangles: basket thermocouple catheter, circles: thermistor catheter.
(a) Casscells et al.4
(b) Stefanadis et al.33
(c) Stefanadis et al.34
(d) Stefanadis et al.38
(e) Toutouzas et al.52
(f) Stefanadis et al.31
(g) Toutouzas et al.13
(h) Stefanadis et al.35
(i) Webster et al.53
(j) Verheye et al.54
(k) Stefanadis et al.37
(l) Schmermund et al.43
(m) Toutouzas et al.36
Heat
A heat source embedded in arterial wall tissue will generate heat, which is transferred to its surroundings, thus causing a temperature difference, which can be detected. The temperature difference depends on a large number of parameters and processes. First of all, it depends on the amount of heat that is generated and on the location and the size of the source. Secondly, on how heat is transferred, and thirdly, on how it is measured.
Heat generation
The mechanism by which the observed heat production of plaques occurs is unknown, but most evidence points towards the high metabolism of macrophages. Macrophages are abundantly present in vulnerable plaques, bordering the atheromatous core, and are located in the fibrous cap, especially near the shoulder regions of the cap2. Speculations have been made with regard to the processes that are involved in the heat generation, including the high metabolic activity of inflammatory cells, the presence of the uncoupling protein 2 (UCP-2) in the macrophages6-8, and lipid endocytosis and conversion by macrophages2. In addition, the action of matrix metalloproteinases (MMPs) secreted by macrophages2 might include exothermal processes.
Macrophage metabolism
The turnover rate for the total ATP content of the macrophage in culture is about 10 times per minute, being an order of magnitude higher than a typical mammalian cell9, which indicates that macrophages have a high metabolic rate10.
Oxygen consumption of the arterial wall increased up to a certain level with increasing degree of atherosclerotic involvement, and a supposed increase in foam cell density11. This might be explained by an increased oxygen consumption of isolated foam cells, which was three times higher than that of isolated smooth muscle cells. However, with increasing intima thickness the available diffusion capacity for oxygen and nutrients, including glucose, to deeper parts of the lesion may become insufficient, which leads to a relative decrease in cellular oxygen consumption of the more diseased atherosclerotic tissue samples11.
Mechanisms responsible for increased energy consumption
Enzymatic extracellular matrix degradation
Microcalorimetric analysis of bacterial collagenase degradation of porcine pericardium tissues revealed that the heat released during degradation correlates well with the degree of tissue degraded12. Therefore, the MMP related breakdown of extracellular matrix in atherosclerotic plaques might also be an exothermal process. Toutouzas et al.13 have demonstrated that patients with acute coronary syndromes show increased MMP-9 concentration, which correlated well with temperature differences between the atherosclerotic plaque and the normal vessel wall.
Lipid metabolism
After the uptake of modified lipids by macrophages through various routes of endocytosis, most of the lipids are finally transported into lysosomes, digested therein, and degraded into amino acids and free cholesterol, which is released into the cytosol and further into the extracellular space. In the cytosol, the free cholesterol is trapped in a continued cycle of esterification and hydrolysis, which is called the cholesterol ester cycle14 that wastes ATP15. The rate of cholesterol esterification is positively correlated with the cholesterol content of arterial segments from atherosclerotic animals16.
Uncoupling proteins
The uncoupling protein (UCP-1), also called thermogenin, is a protein that is found exclusively in brown adipose tissue (BAT) in mammals. Thermogenesis, or heat production, in BAT depends largely on UCP-1 activity17. Of the UCP-1 molecule, a number of homologues exist, of which UCP-2 has been speculated to cause the temperature increase in vulnerable plaques18. Advanced complicated atherosclerotic plaques have shown a dense infiltration of macrophages of which a subpopulation strongly expressed UCP-2. The UCP-2 positive macrophages were associated with oxidized lipids, iNOS and nitrotyrosine and a fraction showed apoptosis. Whether UCP-2 acts thermogenic in any tissue is still under debate19.
Macrophage heat production
Macrophage metabolism, and thus heat generation, can be measured in vitro using microcalorimetry. Heat production values of non-phagocytosing mouse macrophages were shown to be 300 to 2500 x 10-12 cal (0.072 to 10.5 x 10-9 J) per cell per scan, its value decreasing upon increasing cell density20. This corresponds to 0.78 to 6.5 pW per cell. The generated heat was primarily due to glucose metabolism, and glycolysis was a major contributor to the produced heat.
Heat production values of alveolar rabbit macrophages grown in a monolayer were 19.4 ± 3.2 pW per cell21. Adding 20% homologous rabbit serum to the growth medium increased the heat production to 27.0 ± 2.0 pW per cell.
Heat production values of phagocytosing peritoneal mouse macrophages were measured in different experimental conditions22. Mean heat production values for resting cells were 20.6 ± 10.1 and 16.2 ± 3.1 pW per cell. When e.g. lipopolysaccharide bounded high-density polyethylene particles were phagocytosed, these values could rise up to 92 pW per cell, implying an increase of up to five times the basal value.
Heat transfer
Transfer of thermal energy, or heat transfer, between two objects occurs only when the objects are at different temperatures and its direction is from a region of higher temperature to a region of lower temperature. This occurs either by conduction, convection, radiation, or by a combination of these depending on the media involved.
Conduction
Conduction, figure 2(a), is the transfer of heat through a solid or fluid medium due to a temperature gradient by the exchange of molecular kinetic energy during collisions of molecules.
Fig. 2: Heat transfer by (a) conduction, (b) conduction and convection, and (c) radiation and absorption.
Thermal parameters governing this process are the specific heat capacity (how many Joules are needed to raise a certain amount of the material one ºC) and the heat conductivity (the amount of Joules per second (Watts) that pass a certain distance of material driven by a temperature difference of one ºC). Materials can be divided into conductors, for example metals, known for their ability to transfer heat fast, and insulators, for example most plastics, which have low heat conductivity values. Fatty tissue is also known for its insulating properties23, so lipid present in the lipid core of a vulnerable plaque is likely to behave as an insulating material and may obstruct the transfer of heat produced by macrophages to its neighborhood.
Convection
Convection, figure 2(b), is the transfer of heat due to bulk motion of the medium. Heat produced by the vulnerable plaque will be transferred through the wall by means of conduction as described above. Once arrived at the lumen wall it will be transported by the flow of blood. Distal from the heat source, the heat can be transferred back to the lumen wall by conduction.
Parameters influencing the blood flow profile, such as curvature of the vessel and presence of a catheter, will result into changes of temperature distribution. Increase of local flow velocity will decrease the temperatures at the lumen wall24. In addition, pulsatility might effect the measurement, since pulsating axial velocity produces a pulsating temperature distribution25.
In a recently published review26 different ways of heat transfer are also identified. In the paper it is stated that convection is non-significant due to the strong blood flow. Clearly, just because of the strong blood flow in the lumen, convection cannot be neglected24. This can be deduced from the Peclet number, that expresses the ratio of heat distributed by convection to the heat distributed by conduction, which has a value in the order 1x103 for coronary arteries and normal physiological blood flow24.
Radiation and absorption
Heat transfer due to radiation, figure 2(c), is energy transfer via electromagnetic waves. Every object radiates electromagnetic waves, the amount and the wavelength depending on the temperature and its emissivity (how well the object radiates compared to a perfectly radiating black body). Heat transfer by radiation does not require a medium. The radiation in its turn may again be absorbed in a medium, which results in local heating.
In the review26, the radiative heat transfer is considered negligible. It was calculated that an ideally radiating heat source area of 1 mm2, and a temperature difference of 3.3 ºC, emits 22x10-6 W, which is considered extremely small. However, a spherical volume having an area of 1 mm2 could contain 93x103 macrophages of 10x10x10 µm3, which when generating each 20 pW would result in a radiation of 1.4 µW, which would make the 22 µW found in the review not negligible. For much smaller heat sources as described in another paper, having a surface area of 0.05 mm2, and heated to a temperature difference of 3.13 ºC the radiative power is 1.08 µW, which, indeed compared with the heat production value of 5.0x10-3W, is negligible. Apparently, whether it is correct to neglect radiation will depend on the dimensions of the heat source, with a tendency for larger sources to have a higher radiative emission.
Heat detection
Unfortunately, heat production cannot be measured in vivo using calorimetric methods, so other measuring techniques have to be used. One method is measuring temperature. By detecting a higher temperature among a range of so called background temperatures, one is able to locate a spot of higher heat production in the region.
Temperature can be measured by a variety of methods, using electrical, optical, magnetical or other types of sensors. Electrical methods include resistance, thermistor and thermocouple. Optical methods include infrared detection, liquid crystals27 and temperature dependent fluorescence techniques28. Magnetic resonance thermography is a method using the magnetic properties of molecules29. Another method is the use of quartz crystals or microwave thermography30. These methods can be divided into either contact or non-contact methods.
Contact thermography methods require contact between the thermosensitive material of the device and the lumen wall, whereas non-contact temperature methods can do without. Heat detection by a contact method occurs by conduction of heat from the luminal wall to the sensor. When the sensor does not approach the wall close enough, however, the sensor area is exposed to surrounding influences and this will influence the measurement. When designing a contact method, one should be extremely cautious since a vulnerable plaque is easily ruptured. The stress the sensor induces in the lumen wall cannot be too high in order to prevent adverse events.
Non-contact thermographic methods require an absorptive material, which will absorb the electromagnetic radiation emitted by the medium. The choice of this material is wavelength dependent. Many materials used for this purpose are sensitive for absorption in the infrared region. Blood is highly absorptive for most electromagnetic waves of these wavelengths, which makes in vivo detection difficult, since it requires flushing of the artery. Microwave detection could be a better option for in vivo temperature measurements, since microwaves have greater penetration depth for these wavelengths.
Catheter design will influence the temperature measurement, both by material choice as well as its geometric design (shape). As the presence of a catheter in an artery will change the flow profile, and the shape of the catheter is of influence on the profile, presence of a catheter will thus influence the temperature reading.
Previously used catheters and reported studies
The electrical sensors, thermistor and thermocouple, are well known and currently applied in intracoronary thermography catheters. These sensors are very sensitive to thermal changes of the object, but will also be affected by thermal changes of the surroundings of the object. Therefore, these sensors need to be well insulated to optimize the detection of local hot spots. A thermistor needs a high current to achieve accurate readings. However, using a high current will heat the thermistor, which will influence the measurement and this is unwanted. A compromise is thus needed.
The electrical signal is transported from the sensor to the measuring device using metal wires. Metals are known to be very good conductors, so the flow of heat from a hot spot through these wires may significantly lower read out. This is called thermal leakage and it should be minimized.
In vivo validation of intracoronary thermography, and of any other vulnerable plaque detection technique, encounters the fundamental problem that no golden standard to determine vulnerability of a plaque yet exists, although several IVUS based techniques are currently investigated. Currently, standard IVUS is used to determine presence of a plaque. Using this imaging technique, it is not possible to classify the plaque as vulnerable, though.
In this section, the main studies on intracoronary thermography are discussed. The studies are grouped together according to the temperature measuring devices that were applied. The results are discussed in light of the framework presented in the previous section.
Needle thermistor
The needle thermistor consists of a 0.6 mm diameter, 4 cm long needle in which a thermistor is embedded in the tip (figure 3). Its accuracy is 0.1 ºC, and it has a time constant of 0.15 s.
Fig. 3: Needle thermistor (image from www.coleparmer.com).
Cascells et al.4 were the first to detect thermal heterogeneity in human atherosclerotic plaques using this needle thermistor. The measured temperature differences correlated positively with the density of underlying cells, of which most had characteristics of macrophages. Temperature differences of up to 2.2 ºC were reported.
The positive correlation between temperature measurement and macrophages makes it plausible that the heat source consisted of macrophages. If absolute numbers had been given it would have been possible to approximate the heat generation values of macrophages in vulnerable plaques.
The arteries were studied at room temperature within 15 minutes after excision, which has an impact on the heat transfer. This implies that the arteries have had time to cool down. However, the different components of plaques will cool down at different rates due to their differing thermal parameters. In addition, hot spots, currently assumed to be embedded in lipid tissue, cannot transfer their heat easily to their surroundings, since both lipid and air are known for their insulating properties. And blood flow, acting as a cooling liquid in vivo thereby diminishing thermal heterogeneity, is absent in these ex vivo measurements. All these factors might have resulted into greater thermal heterogeneity in these ex vivo measurements than is present in vivo. Since the metal needle, being at room temperature, has a high heat conduction itself, temperature differences of layers deep in the tissue may have been masked by heat leakage through the needle to the environment.
Hydrofoil thermistor catheter
The hydrofoil thermistor catheter (Epiphany Coronary Thermography Catheter, Medispes SW AG) consists of a thermistor embedded in a so-called hydrofoil shaped polyurethane shaft (figure 4). The thermistor has an accuracy of 0.05 ºC, a time constant of 300 ms and is 0.5 mm in size31. A more advanced design includes a balloon to enable occlusion of the vessel32.

Fig. 4: Hydrofoil thermistor catheter (original design A, and modified balloon design B) (image from www.medispes.com).
Stefanadis et al. and Toutouzas et al. have published most of the intracoronary thermography data of human in vivo studies and were the first to do so33, using the hydrofoil thermistor catheter. From their results it was concluded that temperature heterogeneity increases from stable angina patients to unstable angina patients to acute myocardial infarction patients34. It was also concluded that increased temperature heterogeneity is a predictor for an unfavorable outcome after percutaneous coronary interventions31, and that statin treatment reduces thermal heterogeneity in atherosclerotic plaques35,36.
In 20%, 40% and 67% of the stable angina patients, unstable angina patients and acute myocardial infarction patients thermal heterogeneity, and thus a focal heat source, was detected34. The lesion of interest was selected using biplane angiography. Whether the artery was healthy or diseased was verified by standard IVUS. The study shows interesting temperature differences between different patient groups. Using standard IVUS one cannot determine whether a plaque is vulnerable or not, as was described previously. Some might be vulnerable, some not. This might explain why temperature heterogeneity was detected in only a subset of patients. Temperatures were obtained at five different locations at the plaque site. The data show that there was hardly any temperature variation within the plaque, which could mean that the heat producing cells are spread out over the plaque.
Because of the possible convection of heat due to the blood flow, it was suggested that thermal heterogeneity is underestimated in atherosclerotic plaques. Stefanadis et al. studied the cooling effect of blood in effort angina patients37. Temperature differences of atherosclerotic plaques were measured both with blood flow and during full occlusion of the vessel. The vessel was occluded by a balloon filled with saline at 37.0 ºC. Whether this saline temperature was a good choice depends on the temperature of the healthy vessel wall. Temperature differences increased upon full occlusion of the coronary artery and went back to baseline levels after complete flow interruption.
The hydrofoil design of the catheter used to measure temperature was introduced to generate flow-induced forces that would direct the catheter towards the vessel wall. To test whether the sensor makes contact with the wall a 20 MHz ultrasound transducer was used34, which allows to detect gaps between the catheter and the wall exceeding 100 to 200 µm, depending on the spatial resolution of the ultrasound probe.
The thermistor is surrounded by polyurethane, which has a low thermal conductivity (0.251 W/m ºC). It thus acts as an insulator to the thermistor, which makes it a good material for this purpose.
Although the sensitivity of this device may be high, its practical utility may be somewhat restricted regarding the necessity of repeated pullbacks at different angular positions, due to the presence of only one thermistor in the catheter.
Temperature differences are defined as the difference between the maximum temperature measured at the lesion site and the body core (mouth) temperature38. However, the group of control patients show quite large temperature differences, which might explain the relatively large temperature differences for the patient groups.
Of five temperature measurements at the site of healthy vessel wall, the most frequent temperature was used as background temperature and of five temperature measurements at the diseased vessel site, the maximum temperature was designated as the lesion temperature31,34-36. Temperature differences were calculated by subtracting the background temperature from the diseased vessel temperature. This choice might have biased results towards optimistic temperature differences.
Thermistor catheter
The thermistor catheter (Thermography catheter, Thermocore UK Ltd.) consists of 4 thermistors attached to flexible nitinol strips (figure 5). The thermal accuracy of the thermistors is 0.006 ºC and the time constant less than 100 ms39.

Fig. 5: Thermistor catheter (image from Verheye et al.39).
Both Verheye et al. and Diamantopoulos et al. have used the thermistor catheter for their studies. The former concluded that in vivo temperature heterogeneity of rabbit atherosclerotic plaques is determined by plaque composition40, and were thus the first to relate intracoronary temperature measurements to plaque composition. Ex vivo experiments in this study demonstrated the relation between local temperature and local total macrophage mass. Both groups reported on the influence of flow on intracoronary temperature measurements using this catheter39,41.
The ex vivo measurements on atherosclerotic aortas of 3 rabbits using this catheter were performed at room temperature40, which might have influenced heat transfer, and thus the results, in the same way as was described in section “Needle thermistor”, describing the study by Casscells et al.4.
Diamantopoulos et al. also concluded that coronary flow has an effect on the arterial wall temperature41. According to their results, this appears only below a critical threshold of average peak velocity and in a logarithmic fashion. A balloon was inflated with contrast agent at room temperature. This balloon was kept at least 10 mm from the sensor area to reduce influence on the temperature reading. Each flow restriction step lasted 30 s before the restoration of flow was allowed. This appears to be a long time span to be able to maintain a constant flow at which temperature can be measured, since an artery will adapt to flow occlusion by expansion and a hyperemic response. Full occlusion of an artery is manageable, but maintaining a certain constant flow in an artery could be quite troublesome.
Verheye et al.39 concluded that the temperature heterogeneity remained unchanged under normal physiologic flow conditions. In this study, too, it is extremely difficult to control the average flow velocity. When only a minor blood stream passes the balloon, it will wipe away the collected heat. Their results confirm the findings by Diamantopoulos et al.41 since also in this study the relationship between flow velocity and temperature difference was of logarithmic fashion up to a certain flow velocity. This means that temperature differences show a steep drop in the low flow range, whereas the temperature differences remain relatively constant in the high flow range. For velocities above this threshold value, the measured temperature differences remained relatively constant.
The thermistors of the thermistor catheters are brought in contact with the lumen wall after unsheathing the catheter. Nitinol is a nickel-titanium alloy and has a high thermal conductivity (around 10 W/m ºC), which makes it an excellent heat conductor. This will reduce the sensitivity to detect temperature differences, since the blood flowing around the thermistor sensors will cool the nitinol strips on which the thermistors are mounted, and will thus indirectly cool the environment of the thermistors. The very high flexibility of the nitinol strips is needed to prevent vascular wall damage and adverse effects during or after the procedure. Verheye et al. have tested this and reported that the intracoronary thermographic measurements using this catheter were safe and feasible with a similar degree of de-endothelialization as IVUS39.
Basket thermocouple catheter
The controllable basket catheter (Intravascular Thermography Catheter, Volcano Therapeutics Inc.) is made from nitinol wires and contains 9 built in thermocouples (figure 6). At each bend, 2 thermocouples are placed 0.5 mm apart and in the middle wire 1 thermocouple is located to measure blood temperature. Thermal resolution of the thermocouples is 0.001 ºC and the thermal accuracy is 0.02 ºC42.

Fig. 6: Basket thermocouple (image from Schmermund et al.43).
Both Naghavi et al. and Schmermund et al. have used the basket thermocouple catheter42,43. Naghavi et al. have found foci of warmth on the surface of atherosclerotic but not on disease-free regions of femoral arteries of cholesterol-fed dogs. In addition, marked thermal heterogeneity was observed in aortas of atherosclerotic Watanabe rabbits but not in normal rabbits. Schmermund et al. have found temperature heterogeneity in both stable and unstable angina patients, however less pronounced than found by Stefanadis et al.34.
The study by Naghavi et al.42 used both an in vitro and an in vivo model of atherosclerotic plaque. The in vitro model consisted of a tube around which coils of thermal-resistant wires were wrapped to simulate a heat source, placed in a newborn incubator which was kept at 37 ºC. The in vivo model was two animal models, one canine and one rabbit. The study by Schmermund et al.43 was an in vivo patient study.
Both the influence of flow and the effect of luminal narrowing were studied by using the basket thermocouple catheter42 in the in vitro model. With respect to heat transfer, it was found that the cooling effect was proportional to blood flow.
Thermocouple guidewire
A thermocouple is attached on a polymeric sphere covering the tip of a 0.014” guidewire (figure 7) (ThermoCoil System, Imetrx Inc.). A localized bend near the tip of the guidewire causes the tip to contact the lumen wall. The thermocouple guidewire can detect changes in surface temperature with a precision of less than 0.08 ºC and has a time constant of 30 ms.

Fig. 7: A) Details of thermocouple guidewire, (B) Thermocouple guidewire in in vitro set-up (image from Courtney et al.44).
In a well designed in vitro study a relation between flow, or heat transfer by convection, and maximal achievable temperature difference is found for this thermocouple guidewire system44. Both the flow rates and the measured temperature ranges are realistic, based on the in vivo results that have been obtained so far. The in vitro set-up gives a good opportunity to test the catheter intensively and to prove and characterize its function before moving on to in vivo experiments.
The guidewire is connected to a motor driven system and is rotated along a helical path with an average pitch of 2 mm. Since heat sources may be smaller than 2 mm, this hampers the ability to detect all temperature elevations, even though local heat sources will cause a thermally diffuse pattern at the lumen wall24. No experience is obtained in more complex geometries, yet, where non-uniform rotation might cause uncertainties in locating the heat source. In addition, rotation of the tip may be well controlled in straight segments, but will be more difficult to control in curved pieces of artery or in presence of pulsatile flow.
Detection of temperature distributions using a mathematical model
Using a more fundamental approach, Matsui et al. have determined temperature distribution of both a model vessel wall45 and swine aorta46 using thermocouple measurements at defined locations under pulsed laser irradiation and mathematical models. The mathematical model incorporated the temperature changes that occur due to pulsed laser irradiation.
In these articles it is assumed that heat generation is located in the atheromatous core. Currently it is assumed that heat production is related to macrophages, which are located at the shoulders of a vulnerable plaque or infiltrated in the cap or surrounding the lipid core. The location of macrophages is most certainly related to the borders of the atheromatous core, but macrophages might also be present at other locations.
Flow was not considered in the model, so heat transfer from the source to the surroundings was due to conduction and radiation and absorption. Different temperature changes occurred due to the different thermal parameter values and emissivity of the tissues, and the wavelength of radiating light source.
The temperature change that occurs after the laser pulse is dependent on the thermal parameters of the tissue involved. These cannot be determined exactly. These parameter values are needed for the calculations to closely approximate the temperature of the atheromatous core.
Conclusions and recommendations
This review has covered a selection of the results on intracoronary thermography that were published in the past. In addition, it has described the catheters that were used to obtain these results. Both were done from a physics point of view. Using this point of view, it opens new research opportunities in this area.
Previously published results are represented in figure 1. In this figure, it can clearly be seen that the temperature differences that were reported show a decrease over time. The first data points, labeled (a), represent the variation in temperature differences that were found by Casscells et al.4 in their ex vivo study. Due to the lack of detailed knowledge on the difference between the ex vivo set-up of this study versus the in vivo set-up of the studies following this one, it is difficult to clarify these differences in temperatures. The studies labeled with the black squares were all performed using the hydrofoil thermistor catheter. Of these, the study labeled (k) was done proving the influence of flow, which shows higher temperature values at the lumen wall when the vessel was fully occluded, as expected. These values, though, are much lower than the values reported in studies labeled (b) through (h), which suggests that catheter design or study design might have been of influence during measurements for these studies.
Heat source
Data from the studies labeled (i) and (l) are obtained using the basket thermocouple catheter and data labeled (j) are obtained using the thermistor catheter. The values of these data appear to be in agreement with each other, making it plausible that temperature differences of this magnitude, 0 to 0.3 ºC, are most realistically to be expected in vivo, however the temperature differences that have been reported so far are hard to reconcile with the heat production values of macrophages that can be found in literature24.
It is therefore necessary to quantify more accurately the heat generating processes in atherosclerotic plaques before continuing development of temperature measuring techniques. To determine the amount of heat that can be generated by atherosclerotic plaques, one would have to isolate fresh atherosclerotic plaque and study it using calorimetric methods. After this, one could isolate different cells, such as macrophages and foam cells, and/or other substances from the atherosclerotic plaque and subsequently study these selections calorimetrically to determine the exact origin of the heat generation. When a relationship between temperature increases in vulnerable plaques and plaque composition has been determined, then vulnerability of a plaque can be related to temperature measurements.
Some quantitative reasoning may aid to estimate the possible heat production of macrophages from another perspective. The nutritional value of fat equals 38 kJ/g. Considering a 100x100x100 µm3 droplet of fat with a density of 0.900 kg/l, this equals an energy content of 34.2 mJ. This same volume corresponds to 1000 macrophage cells of 10x10x10 µm3. Assuming a heat production of 20 pW per cell, this equals a heat production 20 nW for this volume. If this heat is obtained from burning an amount of fat equal to its own volume, this will require, or last, 20 days. However, heat production values of 20 pW per macrophage appear too low to generate temperature differences that are reported in literature24, implying that fat may be burned in a much shorter time span and that glucose metabolic activity and MMP tissue degradation must also be significant contributors.
Heat transfer
Given the fact that the temperature differences that have been reported most recently are small, it appears mandatory to exclude external thermal influences, such as those from heart muscle heat production and cooling of blood in lungs, or to compensate for them. Evidence for such mechanisms may be derived from several studies, since flow of blood influences the lumen wall temperature measurements37,39,41. Furthermore the location and organization of heat sources are of influence24. For these reasons it is recommended to combine intracoronary temperature measurements with both flow measurements and a vessel wall imaging modality. In this way, localization of a plaque and its morphology can be assessed47,48, flow can be measured separately49, and dedicated imaging and signal processing techniques50,51 could even be added to obtain additional functional parameters that could be used to characterize the plaque.
Heat detection
The catheters that are currently used are based on electrical principles, and need to be insulated very well, as was described in sections ‘Heat detection’ and ‘Previously used catheters and reported studies’. Other methods to measure temperature exist that may prove to be advantageous regarding this matter. Optical equipment, using fiber technology, might be a more appropriate choice for temperature measurement. Combined with heat sensitive fluorescent coatings or liquid crystal coatings this might give a whole new direction in the field of intracoronary thermographic measurements. Another method to measure temperature is using magnetic resonance29, but so far it’s resolution is not sufficiently high. A great advantage is the non-invasive character of this method.
All catheters that are currently used for intracoronary thermography, and that have been described in this review, only sparsely sample the vascular wall. The hydrofoil thermistor design measures temperature at one location, leaving most of the circumference of the vascular wall unexplored. The thermistor catheter and the thermocouple basket catheter measuring at 4 locations still leave over 2 mm gaps of vascular wall in circumferential direction undetermined. The thermocouple guidewire covers the whole circumference, but leaves gaps of 2 mm in the longitudinal direction. If we assume that macrophage clusters are the heat sources, they may be focal and may have sizes smaller than 1 mm. For this reason temperature increases on the vascular wall may be missed and a higher spatial resolution is needed, although the homogeneity of the temperature measurement in the second part of figure 1, labels (i) through (m), counteract this argument.
During the cardiac cycle the position of the catheter moves, so it is difficult to ensure contact at the same location during the full cardiac cycle. Measuring during diastole phase of cardiac cycle could be a solution, but then the measurement needs to be triggered and the thermal response time of the heat transducers must be accordingly short.
Closing remarks
Intracoronary thermography is, despite the number of publications that have already appeared in literature, still in development. Combined, a lot of research has already been done, but many questions are still unanswered. What lacks is the answer to the question “What are we measuring?” Temperature differences exist in coronary arteries in vivo, but it is unclear what causes them. Is it really a temperature difference due to inflammation that has been measured? And what is the contribution of noise and artefacts? External thermal influences must be investigated. Influences of catheter design must be studied intensively. Changes in temperature due to source differences and flow must be known. All of these, and maybe even more, need to be studied before more firm conclusions can be drawn from thermographic data. This in order to more extensively validate intracoronary thermography and to be able to conclude on the usefulness of the method for vulnerable plaque detection.
Acknowledgements
The STW is greatly acknowledged for their financial support (grant RPG.5442).

