Abstract
We designed the ASTAMI trial to investigate effects of intracoronary injection of autologous cells derived from bone marrow (BMC) in acute myocardial infarction (AMI) using robust and comprehensive methods. After six month follow-up, we found no effect of the treatment on global left ventricular function assessed by single photon emission computed tomography (SPECT), echocardiography and magnetic resonance imaging (MRI). However, there was a marked improvement in left ventricular function (LVEF) in both groups, and patients with low LVEF at baseline had the greatest improvement. Our findings are in accordance with findings from similar trials using adequate methods for evaluation of left ventricular function. The placebo procedure used in some studies in this field introduces several confounders, and a placebo-controlled design may not be ideal. We observed no complications related to the cell administration procedure. However, data from another recent trial confirm that there is an increased risk of a repeated coronary intervention. More research is needed before intracoronary administration of BMC for patients with AMI should be applied on large patient populations, for research or clinical purposes.
Introduction
Our interest in cells derived from bone marrow (BMC) for patients with cardiovascular diseases was triggered by the reports from small-animal models that BMC could regenerate myocardium by transdifferentiation, and improve cardiac function after myocardial infarction1,2. The feasibility of intracoronary administration of BMC to patients with acute myocardial infarction (AMI) was demonstrated in early phase I trials3,4. The method seemed safe, and the results indicated improved cardiac function. However, these studies were small and not randomised. Rikshospitalet and Ullevål University Hospitals in Oslo, Norway serve a total population of about 2.5 million people for acute PCI in STEMI. Institute of Immunology at Rikshospitalet Medical Centre has experience with bone marrow transplantation for clinical and research purposes, and the ex vivo cell laboratory is certified for cell production under “good manufacturing practice”, GMP conditions. Thus, we had the necessary patient population and facilities, and in the end of 2002 we designed the autologous stem cell transplantation in acute myocardial infarction (ASTAMI) study, an adequately powered, randomised clinical trial to test the efficacy and safety of BMC therapy for AMI patients5.
Methods
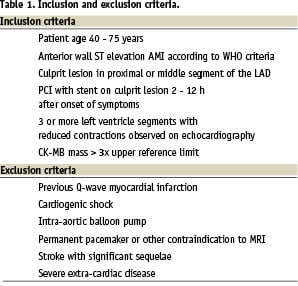
Most STEMI patients treated with PCI in the acute phase have an excellent prognosis with current state-of-the-art treatment. BMC treatment is resource demanding, and if this therapy ever will be a part of routine patient care, it will probably be cost effective only for patients at risk of developing congestive heart failure. Inclusion and exclusion criteria (Table 1) in the ASTAMI study were chosen to obtain a homogeneous patient population with extensive myocardial infarctions well suited for accurate evaluation of left ventricular function. We included only patients with anterior wall infarctions, since this infarct localisation has greater impact on left ventricular function6 and wall motion analysis is most accurate in anterior wall segments7. Different imaging modalities have varying strengths and weaknesses8, and we decided to perform SPECT, echocardiography and MRI for a comprehensive assessment on left ventricular function. Power calculations were based on data obtained with scintigraphy techniques, and SPECT was chosen as the primary imaging modality. We considered a 5% absolute difference between groups of the change in LVEF from baseline to 6 months to be a clinically important effect of this highly invasive approach, and with 80% power and an α of 0.05, we needed 45 patients in each group. To allow for some drop-outs, we decided to include a total number of 100 patients.
BMC represent a heterogeneous cell population. The different precursor and stem cells like haematopoetic stem cells (HSC), endothelial precursor cells (EPC), mesenchymal stem cells (MSC), side population (SP) are examples of cell subsets of mononuclear cells derived from bone marrow (mBMC) that may have a therapeutic potential. Since it was, and still is, unknown which cell subset(s) could participate in cardiac repair after AMI, we decided to use unfractioned mBMC in the ASTAMI study. Common to standard technique for isolation of mononuclear cells, we used the well-proven ficoll-hypaque (Lymphoprep, Axis-Shield) density gradient centrifugation. The approval of the Norwegian Medicines Agency restricted the use of artificial cell-culture mediums for cell processing, and only autologous products were used in addition to ficoll-hypaque and saline. Cell quality was assessed according to pre-specified criteria5 immediately before intracoronary injection. The PCI stop-flow technique described by Strauer et al was used for cell injections3. We decided to perform cell injections 4 to 8 days after the AMI, based on an “educated guess” that this would be the ideal time-point. We supposed administration of cells earlier would be disadvantageous due to the extensive inflammatory response, and later due to reduced levels of homing and transdifferentiation factors from the infarcted tissue. For ethical reasons, bone marrow aspiration and sham intracoronary injections were not performed in the control group.
Results
Patient characteristics were well matched (Table 2). After processing 50 ml of bone marrow, the number of mBMC for intracoronary injection was 68x106 (interquartile range 54 to 130x106). Cell viability was always over 90%. The phenotypic characteristics of certain stem or progenitor cells are provided on www.nejm.org9. The numbers of CD34+ or CD133+ cells per number of mBMC or per volume aspirate were lower than age-matched controls (unpublished data), indicating that AMI patients have changes in the cellular content of the bone marrow, which may affect the therapeutic potential of mBMC.
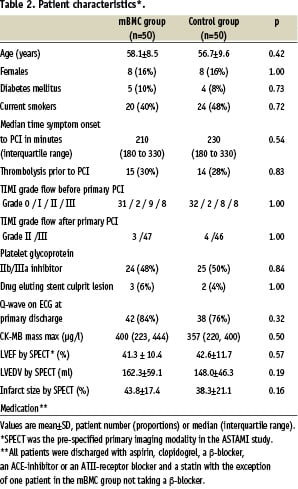
In the 47 patients who received intracoronary mBMC, 34 (72%) had chest pain and 36 (77%) had ischaemic ECG-changes during balloon inflations. Except for one patient who had a rise in troponin I of 0.05 µg/L, all patients had a decline in troponins the day after the cell injections. The rise in CK-MB in 8 (17%) patients was probably related to the bone marrow aspiration, since 5 of these patients also had elevated CK levels after bone marrow aspiration. No patients had re-infarctions or other complications in relation to the cell injection procedure. We found a similar improvement in left ventricular function in the randomised groups9 (Table 3). By dichotomising the patients according to the median value for LVEF at baseline (43.0% by SPECT), we found that the patients with low LVEF at baseline had a greater increase in LVEF than the patients with high baseline LVEF, independent of treatment allocation (Figure 1). Infarct size was reduced with approximately 10% points independent of infarct size at baseline and treatment allocation.
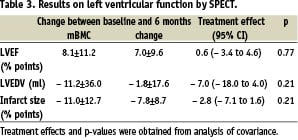
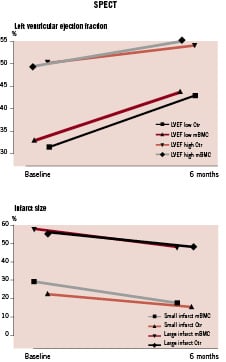
Figure 1. Effects of baseline values on the change in LVEF and infarct size. The study population was dichotomised according to the median value for baseline LVEF and infarct size respectively. Symbols show mean values.
Discussion
The ASTAMI study was obviously a negative trial with regard to the primary endpoint, which was the change in LVEF from baseline to 6 months follow-up. It has been speculated whether the number of mBMC and various stem and progenitor cells were insufficient to induce clinical improvement. Furthermore, it has been proposed that our cell preparations impaired cell quality10. This is unlikely for the following reasons: a) the cell number in ASTAMI is comparable to other studies11,12; b) cell numbers have not been found to correlate with improvement in LVEF9,13,14; c) injected cells in our study always had a high viability; and d) mBMC produced by our procedure had normal content of functionally intact haematopoetic progenitor cells like CFU- GM and BFU-E (unpublished data). Furthermore, Lymphoprep, the ficoll-hypaque medium used in ASTAMI is similar to the ficoll-hypaque used in e.g. REPAIR-AMI.
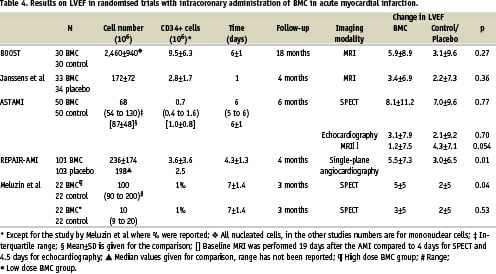
More likely, our results reflect the limited effect on myocardial function by intracoronary injection of BMC a few days after a reperfused AMI. This finding is in agreement with results of other adequately sized randomised trials using appropriate methods for assessment of LVEF15,16 (Table 4). The study by Meluzin et al was rather small12, and demonstrated a modest effect on LVEF in the high dose group, where similar cell numbers as in ASTAMI were injected. In the REPAIR-AMI study17, also demonstrating a small effect on LVEF, interpretation of the results is hampered by their use of single-plane angiocardiography, which is an particularly inaccurate method in these patients with regional wall motion abnormalities18. In REPAIR-AMI, patients with low LVEF treated with mBMC improved LVEF with 7.5% compared to 2.5% in the placebo group17, suggesting that the therapy is particularly useful for patients with large infarcts. The results of the ASTAMI trial confirm that patients with low LVEF at baseline improve LVEF more than the patients with high LVEF. However, this was independent of treatment allocation. In our opinion, the most remarkable finding in this sub-group analysis in REPAIR-AMI, is the small improvement in their placebo group.
We decided not to perform bone marrow aspiration and sham intracoronary injection in the control group. Even though a placebo controlled trial is considered the gold standard to assess an intervention, it should be discussed whether this approach is valid for these interventional trials. It is important to note that the placebo-procedures used in the study by Janssens et al and in REPAIR-AMI are not indifferent. Cytokine release can be induced by bone marrow aspiration19 as well as by PCI20. Repeated balloon occlusion of the infarct-related coronary artery can also affect the infarct process21, introducing several confounders. In addition, patient safety is paramount. There is a small but definite increased risk by repeated coronary intervention in these patients, as recently reported by Meluzin et al12. Out of 44 patients in that study, one had stent thrombosis and another had coronary artery dissection as direct complications to this procedure.
Some researchers advocate for large-scale trials to investigate effects on morbidity and mortality10,17. In our opinion, the results of the available randomised studies in this field do not support this notion. There are still many important unresolved issues in this field, and proceeding too fast towards clinical application without sound evidence from basic and clinical science may set the field back. Among urgent questions that need to be answered are: Which cells should be used? How should they be prepared? Which patients will benefit most? How should cells be administered? What is the ideal timing for cell administration ? Should treatment be repeated? What surrogate end-points are most appropriate for small to medium-scaled trials? These questions should be adequately answered before large scale trials in this field are initiated. Documented effects on quality of life, functional capacity, morbidity and mortality will finally be needed before cell therapy can be recommended for AMI patients in clinical practice.

