A variety of non-coronary percutaneous interventional therapeutic approaches have been developed during the last two decades. Many of these, e. g. device closure of inter-atrial communications, are to a certain extent dependent on echocardiographic guidance. Transoesophageal echocardiography (TEE) is well established and provides exceptional high-resolution images, but the need to keep patients in the supine position during percutaneous interventions limits its usefulness. Continuous TEE monitoring is highly desirable for precise device deployment, but the probe is usually only tolerated if patients remain sedated or under general anaesthesia. As summarised by S. Vaina et al., intracardiac echocardiography (ICE), also known as intracardiac phased-array imaging, has become an alternative to safely guide a multitude of complex interventional procedures. Up to now, however, there is no evidence that ICE guidance can improve the safety of these procedures.
One would expect the more sophisticated interventional procedures to derive more benefits from ICE. Thus, there is great potential for ICE in percutaneous valve implantation techniques and other catheter based “valvular repair” procedures currently under development. Especially transcatheter mitral annuloplasty techniques appear promising, since inoperable patients with congestive heart failure are prone to develop considerable relative mitral regurgitation.
The AcuNav®-catheter is not wire-guided and therefore different from conventional intravascular ultrasound (IVUS) catheters. This may present a potential risk. In our experience, the risk is very low provided the ICE-catheter is handled with caution1. That includes use of a long access sheath and fluoroscopy, two recommended precautions to safely guide the catheter into the heart. Currently, European Community approval (CE-mark) has only been granted for transvenous and right heart applications. The large probe size (10 F) mandates an access sheath of 11 F, thus hampering the use in small children and any transarterial approach.
The introduction of the new 8 F AcuNav®-catheter will facilitate ICE and probably increase safety and patient comfort. The initial clinical experience in paediatric cardiology is promising2. The catheter has evolved: It possesses the unlimited echocardiographic capabilities of its predecessor but is even longer (insertable length: 110 cm), thus opening up new opportunities for intraluminal phased-array imaging (IPAI) in aortic diseases. As recently reported, TEE adds incremental information to safely guide stent-graft placement in type-B aortic dissection. In addition, IVUS is considered helpful in patients with complex dissection including abdominal extension3. Sonographic approaches may help lower the current complication rate of percutaneous stent-graft implantation of about 11.8±3.7% (higher in emergency cases)4.
With respect to aortic diseases, the main limitation of IVUS is attributed to its inability to perform Doppler analysis. TEE is restricted to visualisation of the thoracic aorta. Moreover, flow detection by TEE is also limited if the flow is not aligned with the Doppler beam. In consequence, the recognition of flow from the true to the false lumen and vice versa by TEE depends on the alignment of the dissection flap. Peri-interventional sonographic diagnostic methods need to resolve which lumen supplies the side branches, a difficult feat with TEE and even IVUS images. Thus, the necessity to navigate the aorta with the AcuNav®-catheter turns from a disadvantage into an advantage, since the Doppler-beam can be aligned with any flow between true and false lumen (see cover image) and with blood flow into small branches. Thus, IPAI has great potential to become a tool to effectively guide aortic stent-graft implantation in order to close all entries. First case reports also suggest that IPAI is capable of safely guiding diagnostic and therapeutic intra-aortic procedures in aortic diseases5,6.
The price of approximately e 3,000 for one AcuNav®-catheter remains an important shortcoming. But today, AcuNav® is no longer a disposable device. The lumenless catheter can be resterilised with gas. Vanguard AG Medical Services for Europe (Berlin, Germany) is an approved CE-certified company licensed to resterilise AcuNav®-catheters. The company guarantees technical and medical safety and compliance with appropriate legal regulations governing health care products. After more than two years of experience at the West-German Heart Center in Essen (University Duisburg-Essen), resterilisation can be recommended as a safe procedure, which permits each catheter to be reused approximately three times. Although the image quality may slightly deteriorate with resterilisation, each catheter can eventually be used four times, lowering the costs for one use to clearly less than e 1,000, including expenses for resterilisation.
Safe utilisation of sophisticated therapeutic methods inside the heart or the great vessels remains challenging. Nowadays, ICE and IPAI are about to shed new light on the feasibility of non-coronary interventions. Routine simultaneous echocardiographic and sonographic imaging may further the development of interventional therapies. Procedures can be facilitated by combining sonographic and fluoroscopic images (image-in-image viewing). The highest benefit from invasive sonographic techniques such as ICE and IPAI can only be gained if the examiner is highly familiar with echocardiography, a prerequisite for today’s modern interventionalist.
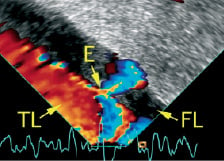
Figure 1. Intraluminal phased-array imaging in type-B aortic dissection with the AcuNav®-catheter inside the true lumen shows small entry supplying the false lumen with partial thrombosis. Entry: E, false lumen: FL, true lumen: TL

