Introduction
Since the clinical introduction of the first drug-eluting stent (DES), in April 2002, this breakthrough technology, including the sirolimus-eluting coronary stent (SES), the paclitaxel-eluting stents (PES), and many other new investigational agents like everolimus-, zotarolimus-, and tacrolimus-eluting stents, has been tested extensively for its main purpose of preventing restenosis. Their effectiveness in reducing restenosis and the associated target vessel revascularisation (TVR) is not in dispute; however the long term safety of these devices is the subject of ongoing debate.1-3 Especially the increased risk of potentially fatal late thrombotic events associated with the ‘off-label’ use of DES in complex lesions and specific patient subsets is of great concern.4
Accurately determining the incidence of adverse clinical events, especially in the case of rare but catastrophic occurrences such as stent thrombosis (ST), is a challenge for all constituencies, including regulatory authorities.5 It is clear that detailed analysis of published trials in this field is critical for understanding the conclusions. The use of uniform, hierarchical definitions should help in evaluating risks across studies. In an effort to enable meaningful comparisons between clinical trials, the Academic Research Consortium (ARC), formed in 2006, proposed standardised definitions for clinical endpoints in coronary stent investigations and made them available to any and all interested parties via peer-reviewed publication.6 ARC consisted of academic representatives in the field of invasive cardiology, academic research organisations active in the field, as well as members of industry and regulatory bodies. The ARC consensus definitions were developed completely separate from any considerations concerning potential applications of these definitions. They may serve as a lighthouse, a point of reference, for the medical community wishing to compare studies on DES technology and as a toe in the water when exploring the safety profile of new PCI devices or evaluation of more complex patients or lesion subsets.
In this manuscript we intend to introduce to the interested reader a comprehensive flow scheme for stent thrombosis (ST) adjudication following the ARC definitions. It may provide a visual guide to understand the numbers reported in tables of published manuscripts or abstracts. We implemented this tool while re-adjudicating potential stent thrombosis events from the ARTS II trial according to the ARC definitions. The results of this re-adjudication were published earlier in detail5. This exercise allowed for a critical appraisal of the ARC definitions. Specific elements of the definitions merit emphasis, clarification or possibly even a correction at some point. Finally, we will make a strong case for the importance of detailed clinical data, including a complete narrative summary to facilitate event adjudication.
The spirit of the ARC consensus definitions
The ARC definitions provide a conceptual framework for the design, analysis and reporting of clinical trials evaluating the effectiveness and safety of DES in an elective percutaneous coronary intervention (PCI) population, as well as the pharmaceutical adjuncts related to this technology. They are based on considerations ranging from historical legacy to key pathophysiologic mechanisms and relevance to clinical interpretability.5
Ultimately, ARC stresses the importance of standard reporting of clinical outcomes according to both a device related composite, defined as cardiac death, myocardial infarction (MI) involving the target vessel territory or target lesion revascularisation (TLR), and a patient-related composite that assesses the net impact of device treatment on overall clinical outcome, defined as all cause death, any MI, or any repeat revascularisation procedure.
While either of these composites can be argued as appropriate primary clinical endpoints for a DES clinical trial, since they represent measurable outcomes that reflect overall safety and effectiveness and occur with sufficient frequency to provide adequate statistical power, the impact of the individual components of either composite or other safety outcomes such as stent thrombosis has been more difficult to measure and created substantial controversy.
In the case of stent thrombosis in particular, much of the controversy has been fuelled by inadequate and non-standardised clinical definitions, leading many to conclude that only the overall effect on death or MI should be considered. We believe this is problematic since small, but possibly significant differences in stent thrombosis may not have a quantifiable effect on death, myocardial infarction (MI), target lesion revascularisation (TLR) or the composite outcomes. Nevertheless, it is important to quantify differences in stent thrombosis in order to understand possible biologic differences between different DES or bare metal stents (BMS), even if balanced by other causes of death, MI, or repeat revascularisation.
The ARC acknowledges three temporal categories of ST to imply differences in the contribution of the various pathophysiologic processes during each of the intervals. A tri-level of certainty classification is introduced.5 The highest level of evidence of ST requires signs of (acute) myocardial ischaemia with either angiographic7 or autopsy confirmation at any time following the index procedure. The categories of probable and possible stent thrombosis add sensitivity to the ST-definition and should also be reported.
For practicality, we visualised the adjudication process for the different categories of certainty in a comprehensive algorithm (Figure 1). Aligned with the ARC definitions, this flow-diagram is dynamic by nature and may need specification according to specific populations or type of lesions studied. The quality of the clinical adjudication process and the utility of these categories strongly depend on the quality of the data available to the adjudication committee.
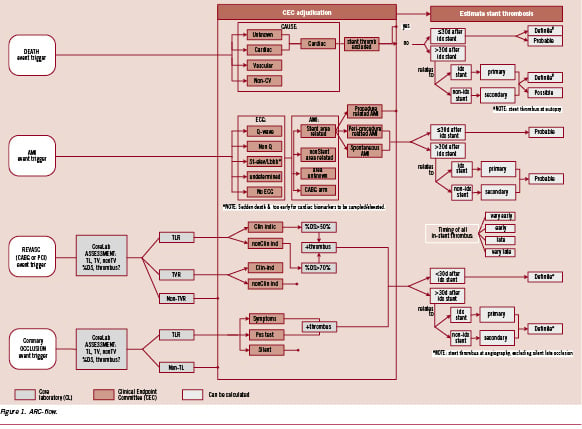
Beyond any reasonable doubt
In quantitative clinical research, investigators use stories to investigate hypotheses that are conditioned by prior scientific knowledge and by their own experience and biases. Despite the potential biases in individual accounts, clinical trials rely on patient stories to provide otherwise unobtainable data about the natural history of diseases, the exposures that cause them, and the effectiveness of treatment. Each story is only provisionally informative until it is corroborated by consistency of findings and confirmed by the best available clinical evidence. These may consist of any patient source documents reporting investigator initiated or protocol driven diagnostic test results (laboratory exams, standard 12-lead electrocardiogram [ECG], angiograms, autopsy reports e.g.). Clinical autopsies are vital to establish the true cause of death and should be strongly encouraged. Quantitative pre-adjudication of coronary angiograms for lesion localisation, severity and visible intra-coronary thrombus by an independent quantitative coronary angiographic (QCA) core laboratory may significantly contribute to the quality of this process.
The clinical adjudication process is highly specific, being driven by the application of pre-specified criteria for event definitions, and does not always allow full elucidation of the pathogenesis of device related events based only on the individual case report form (CRF) responses. With the example of probable and possible stent-thrombosis adjudication, we make the methodological case for a (systematic) illness narrative inquiry as a unique means to get inside the history of a clinical event. To avoid any semantic confusion and to allow optimal implementation of information feedback loops, the exact wording, the use of consistent definitions and the structure used in narrative writing are crucial.
As indicated in the flow diagram a detailed narrative inquiry focusing on ST was performed using the three patient oriented composite safety endpoints (all cause mortality, myocardial infarction and any repeat revascularisation) as well as angiographic vessel occlusion in the absence of one of these events. The latter is not de facto evidence of ST, but should stimulate detailed re-evaluation of the full patient files for any evidence to implicate the presence of ST.
Death
“All deaths are considered cardiac unless an unequivocal non-cardiac cause can be established.”
Cardiac deaths should include all events related to cardiac diagnosis, a complication of the procedure, treatment for a complication of the procedure (restenosis, e.g.) or unexplained causes. Considering the latter, any death during the follow-up period that can not be clearly attributed to a non-cardiac cause will be adjudicated as cardiac death based on a worse case scenario. According to the ARC definition, a patient who dies following a bleeding complication while on concomitant dual anti-platelet therapy following DES-implantation will be adjudicated as cardiac death. However, if dual antiplatelet therapy was not given per-protocol but indicated for other disease (cerebrovascular disease e.g.) this event would be adjudicated as a non-cardiac death.
According to the ARC ST definitions, any death during follow-up of truly unknown cause will be adjudicated as probable (if within 30 days) or possible (beyond 30 days) stent thrombosis using the same logic as above for cardiac death. For this process to have the highest level of specificity, it is crucial for the adjudication committee to have the best available clinical information to determine whether death is clearly not cardiac related or if cardiac is most likely not related to stent thrombosis. In the absence of these data, any unexpected death even in patients with coexisting potentially fatal non cardiac causes (e.g. cancer, infection) should be classified as cardiac. Figure 2 shows an example of a coronary thrombus aspirate following a subacute ST in a patient suffering a myelodysplastic syndrome with a rapid course of progression of neoplastic haematopoiesis terminating in a acute myeloid leukaemia.
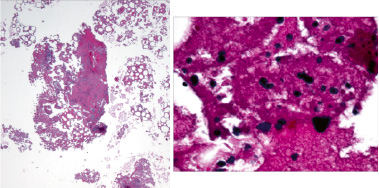
Figure 2. Thrombectomy aspirate containing a fibrin and platelet-rich thrombus with moderate inflammatory cell infiltrate composed of neutrophils and chronic inflammation including lymphocytes with few immature granulocytes. The scattered immature granulocytes in the aspirate consistent with the patient’s diagnosis of refractory anemia with excess blasts (Higher magnification at right).(courtesy of E.R. Ladich, R. Virmani, CVPath Institute, Gaithersburg, MD, USA)
This issue becomes more critical in the late follow-up period when there is a higher frequency of death and details are more likely to be inadequate for specifying a cause. Indeed, during application of the ARC definitions we have found that late deaths are often unexplained, resulting in assignment of a cardiac cause and attribution to possible ST. This results in what is likely a falsely elevated rate of possible ST and the potential to dilute real differences in definite or probable ST, and has led to the suggestion that only definite or probable ST be reported. We believe this is also a concern as it clearly under reports true late, and very late, ST rates. Indeed, it may be clear that an unwitnessed, overnight death nine months following a DES implantation in an octogenarian should be valued differently from a sudden cardiac death in a 56 years old patient who stopped his dual anti-platelet therapy six days earlier for a planned orthopaedic intervention while working in his garden three months after DES implantation.
Acute myocardial infarction
Table 1 highlights some particular elements from the recently published universal definition of myocardial infarction important for (drug-eluting) stent evaluations. The diagnosis of reinfarction (/infarct extension) in the setting of a PCI is not possible in the presence of unstable or declining troponin levels. In this setting the importance of obtaining baseline biomarkers to exclude elevation prior to the index procedure should be emphasised. Moreover, we like to stress that the reinfarction definition consist of two key components. As indicated in Table 1 there should be a 20% rise in troponin level three to six hours post-procedure as compared to baseline. Less clear from the original manuscript8, the CK-MB or troponin rise should bring its value to at least three times above the URL.
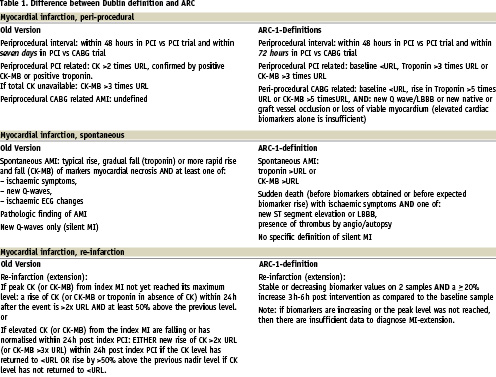
Although a silent MI is not explicitly defined by ARC, any new pathologic Q waves compared to the pre-procedural ECG at any stage during follow-up may define an interval myocardial infarction and suggests its anatomic location. Blinded core laboratory readings and the use of relevant ECGs in event adjudication are strongly recommended. Finally, the diagnosis of spontaneous MI is possible in case of sudden death with ischaemic symptoms and ECG signs of transmural ischaemia (ST segment elevation, new LBBB) or proven vessel thrombus (angiography, autopsy).
Procedural (thrombotic) complications (dissection, guide-catheter [wire] thrombosis, e.g.) may occur during the index study procedure (primary) or during target lesion revascularisation (secondary). A PCI procedure ends when the guiding catheter is removed and the patient leaves the catheterisation laboratory. Thrombotic complications evolving during this time frame will not trigger a (device related) stent thrombosis event according to the ARC definitions.
Importantly, also a (biochemical) periprocedural myocardial infarction (<48 hours of the index procedure), in the absence of any angiographic or pathological confirmation of ST or a recurrent acute ischaemic event, will not connote a probable ST event. The latter reflects our intention to avoid diluting a potential real difference in ST events with the use of an overly sensitive definition that include cases of myocardial infarction due to periprocedural complications (distal embolisation, side-branch occlusion, e.g.). In the periprocedural period (after removal of the guiding catheter up to 48 hours) the element of a new (acute) ischemic event is critical when considering a probable ST. Any new ST-segment elevation myocardial infarction in the region of the index procedure may be related to ST. So any spontaneous ST-segment elevation myocardial infarction in the region of the index study procedure may be related to stent thrombosis. For those patients treated with thrombolytics, the presence of residual thrombus in the stented region triggers the diagnosis of definite ST. In the absence of any residual vessel thrombus and in the absence of any other culprit lesion in the target vessel, ST becomes probable according to the ARC definitions if accompanied by elevated cardiac biomarkers. However, any new ST-segment elevation myocardial infraction post PCI infarction, in the absence of angiography or histology confirmation, is considered a probable stent thrombosis.
Repeat revascularisation procedures
“Clear and consistent definition of target lesion revascularisation (TLR) is crucial to understanding variations in DES effectiveness.”
Any re-intervention procedure that “touches” the stented area is defined as TLR. ARC criteria for TLR are intended to define procedures that are performed for clinically significant re-narrowing. The adjudication process as to the clinical need of a TLR is based on two fundamental components 1) symptoms or any functional evidence of ischaemia, and 2) lesion severity > 50% diameter stenosis determined by an independent quantitative coronary angiographic core laboratory. Ideally the evaluation for clinical signs should be performed by the investigator “prospectively”, at a point in time prior to repeat angiogram. However, a TLR or target vessel revascularisation (TVR) for a diameter stenosis > 70% in the absence of the above mentioned ischaemic signs or symptoms is also considered clinically indicated.
An important lesson learned from previous DES studies is the influence of study design on reintervention rates, in particular, through the use and the timing of protocol-mandated angiography that encourages “occulo-stenotic” interventions with their attendant complications.9 This reflex may be even more pronounced when treating more complex lesions like bifurcation lesions.10 The latter may be of concern in the light of the increased procedural complexity and of the trend towards a higher rate of myocardial infarction in the bifurcation group.11 Although for existing studies the independence of such evaluations may not be possible, for future studies, the completion of clinical evaluations before protocol angiography or the separation of angiographic follow-up studies from clinical studies may be useful.
In these cases we can concur to a strategy introducing invasive functional diagnostic testing as a tool for adjudication as to clinical need.12 Along with this reasoning, any investigator/institution driven, non-protocol mandated, scheduled repeat angiograms in clinically stable patients, as encountered during ARTS-II, are problematic, and should be avoided, in the sense they may induce a bias towards increased TLR (“oculo-stenotic reflex”)13.
Occlusion
A vessel occlusion is the absence of any antegrade luminal passage of contrast dye distal of the lesion of interest (TIMI flow 0-I). The incidental angiographic documentation of stent occlusion in the absence of clinical signs or symptoms is not considered a confirmed (definite) stent thrombosis, since in the absence of a clinical event an abrupt occlusion is less likely than gradual re-narrowing due to restenosis. However, any silent (stent) occlusion should stimulate a detailed investigation of the patient source documents for any indication of spontaneous myocardial infarction (new Q waves, wall motion abnormalities, e.g.) in the area of the implanted stent. As indicated the latter may indicate a probable stent thrombosis.
Censoring analysis?
“Making every event count by counting every event”
Clinical event analysis in randomised clinical trials (RCTs) of DES requires careful scientific and statistical consideration. In case of ST comparisons between bare metal stents (BMS) and DES, early pivotal study designs censored or excluded patients from a subsequent ST event after an intervening reintervention for restenosis. More recent re-evaluations have noted that this analysis plan may bias perception of BMS safety (because of higher restenosis rates and more censored patients) relative to DES. Indeed, we have observed that definite or probable ST is more frequent after treatment of restenosis.14 A dilemma exists in this case as whether to attribute stent thrombosis to the initial treatment strategy (“intention to treat”) or the intervening treatment of restenosis (“as treated”). Elimination of such censorship actually would produce a more theoretically rigorous “intent-to-treat” analysis plan. This observation also adds further evidence against prior assumptions of restenosis as a benign clinical entity.15 Re-intervention for restenosis is often complex and carries an elevated risk for procedural complications, morbidity and mortality.
Reporting stent thrombosis
In the process of adjudicating stent thrombosis events, individual patients may sustain more than one indicator from the algorithm in Figure 1 for the same ST event or they may sustain subsequent ST resulting in > 1 ST during the follow-up period. We propose reporting stent thrombosis according to actual number of unique ST events, assigning each unique event to the highest level of certainty and not counting multiple levels of certainty for the same event. For example, if a patient presents with MI and does not undergo angiography initially but several days later does have angiography demonstrating thrombotic occlusion in the absence of a new clinical event, we propose this be reported as a single definite ST rather than probable and definite. On the other hand, patients with discrete scenarios consistent with ST should be counted as multiple events according to the level of certainty for each event. In the analysis, we propose a hierarchical report, identifying the number of patients with any event, number with >1 unique ST, and the number identified according to definite, probable, and possible level of certainty. This principle is illustrated in Figure 3 of the ARTS-II three-year follow-up paper previously published in EuroIntervention.14
Time of follow-up
The issue of duration of follow-up is important. Ideally, we desire long-term follow-up, but this needs to be balanced against the knowledge that as time advances the probability increases that events are related to disease progression rather than complications of treatment of the original lesion. It is important to assess these events in relation to the overall clinical outcome as with the proposed patient-oriented composite. But, an effort to attribute these very late events to ongoing risk for ST loses specificity after some point and may again dilute real differences assessed at earlier times.
Conclusion
The ARC-1 met the goal of developing standardised endpoints focussed on safety and effectiveness of (investigational) DES platforms in stable coronary disease patients with the de novo lesions. The adoption of a single set of consensus definitions reflecting possible, probable, and definite stent thrombosis is useful, even with the realisation that limitations of these definitions include the variability of sensitivity/specificity, depending on how they are applied and on the quality of the clinical source data. “Clinical narrative competence” is crucial to the quality of the clinical event adjudication. The ARC-1-definitions do not cover every situation. In the near future, specifications will be necessary addressing individual complex patient and lesion groups.

