Introduction
Increasingly, many healthcare systems require cost-efficacy data to support the use of therapies they are planning to fund to ensure they are worth any extra cost incurred. Health economics is the discipline that can help us to develop the methodology and judgement systems to make such decisions, particularly with respect to technologies such as drug-eluting stents (DES) that bring undoubted benefits, but at clearly higher acquisition costs than bare metal stents (BMS). Despite the doubts of many clinicians about the validity of health economics and their concerns regarding what they regard as interference in clinical decision making, it is clear that in any cost constrained health care system not all treatments can be provided to all patients and that clinical benefit in relation to value for money needs to be the mix somewhere. It is vital therefore that physicians embrace the concept of health economics and engage with the process when it involves their discipline or a therapeutic modality that they have involvement in.
Any economic analysis has to appropriately model the difference in costs between the established older therapy and new therapy that is replacing it and any difference in effectiveness of the treatments being compared. In the case of DES, the additional acquisition cost of the DES can be partly offset by avoiding costs of repeat revascularisation that would have been incurred had the patient been treated with BMS. Thus, the incremental cost of DES can be calculated as the difference between the extra acquisition cost and the downstream avoided costs. This basic principle applies fundamentally to all technologies that are designed or have been shown to provide clinical benefits compared with those that are already available.
The definition of effectiveness in health economic terms is more complex and an increasing number of bodies that examine cost-effectiveness, such as the UK’s National Institute for Health and Clinical Excellence (NICE), have adopted the quality-adjusted life year (QALY) as the measure of effectiveness1. It is important to understand what a QALY actually is. It is a description of the longevity of a patient, but adjusted to take account of the state of well-being or quality-of-life (QoL) that the patient experiences. QoL can be measured using standard questionnaires such as the EQ-5D and the resulting scores can be converted into QALYs. The QALY thus reflects the health state and the time spent in that state. It is important to understand that a QoL score is not in itself a QALY. A QoL score of 1 equates to perfect health whilst death has a QoL score of 0. Different health states have different QoL scores, so for example major stroke has a QoL of 0.35 and pre-PCI patients who are symptomatic with angina, 0.692. For a patient who has been undergone PCI the QoL score is 0.84 (2), not “1” because the patient is not in perfect health. The development of symptomatic restenosis leads by consensus to a QoL loss of 0.15 (0.84 – 0.69). Patients remain in this state of QoL loss until their symptoms are relieved, i.e., until their repeat procedure which is dependant on waiting and referral times. For the UK this was calculated as being 0.482 of a year. Thus the QALY loss due to symptomatic restenosis and the loss that DES with their extra cost is seeking to avoid is 0.15 ⋅ 0.482=0.0723. However it is more complex than that since not all BMS patients restenose. If the absolute risk of repeat revascularisation is 13% and this risk is reduced by DES by 70%, then the absolute reduction is 9.1%. Thus the QALY gain from using a DES is 0.0723 ⋅ 0.091=0.0066. This is a conservative estimate because it does not include any QoL loss due to the procedure itself.
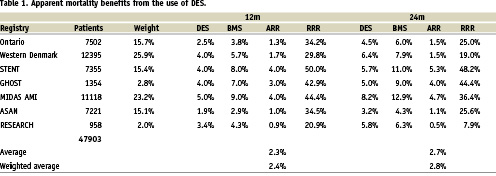
In the overall scheme of cost effectiveness, this QALY gain is very small since it is based on reductions in symptom level rather than in mortality. An increasing number of registries are however reporting mortality reductions associated with DES and our own preliminary analysis of 10 registries suggests that there could be as much as a 1.37% weighted mean absolute mortality benefit for DES at 12 months (Table 1). This result requires confirmation as we have, in some cases, had to estimate event rates from survival curves. However, even using these estimates, it is interesting to see what the QALY gain associated with this mortality gain would be. If death is assumed to occur from the health state associated with angina, the QALY loss due to death over one year would be 0.69 ⋅ 0.0137=0.0094 (compared to TLR QALY loss of 0.0071 for restenosis). This is 1.3 times the QALY loss that can be gained by the reduction in repeat revascularisation. If this were taken into account in economic modelling, it would greatly enhance the cost effectiveness of DES. Currently however, Health Technology Assessment agencies have not considered the impact of this potential mortality benefit, merely the restenosis benefit but may be encouraged to do so in the future.
The ‘effectiveness’ element of the cost effectiveness calculation is thus the QALYs gained by, in this example, the use of DES compared with BMS. This incremental effectiveness, when divided into the incremental cost of DES, gives an incremental cost-effectiveness ratio (ICER) that can be used in decision-making.
The importance of reliable data
An economic analysis is only as good as the data that are used to calculate the ICER. Not every variable in a treatment pathway will have a material impact on the overall cost-effectiveness result, thus it is important to identify those which are likely to. In the case of DES, the absolute risk of repeat revascularisation, the risk reduction derived from DES, and the price premium of DES over BMS are critical factors. The time horizon of the analysis is also important as a 1-year horizon may be appropriate to capture the costs and benefits associated with avoidance of repeat revascularisation, but reductions in mortality or MI may be needed to be considered over a longer time period. Clearly, QALYs accumulate year on year when there is a mortality benefit, by the cumulative avoidance of death.
The BASKET trial3 illustrates several points with respect to the appropriateness of trial data for use in economic modelling. Firstly, BASKET was conceived as a prospective cost-effectiveness study in the setting of routine clinical practice and is so far the only DES trial to specify cost-effectiveness as the primary endpoint. One of the limitations of using trial data to populate economic models is that the restricted inclusion criteria can make it difficult for trial-based cost-effectiveness to accurately represents real-world cost-effectiveness. BASKET overcame this problem by recruiting unselected patients from routine clinical practice.
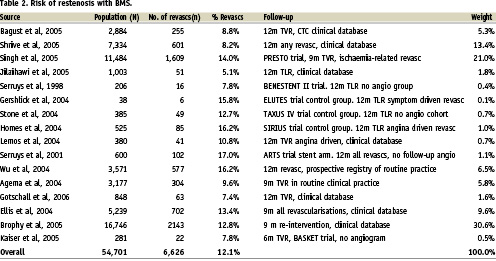
Many DES trials, for understandable reasons, include protocol-mandated angiographic follow-up that would not normally occur in routine practice, where revascularisation is clinical driven. In this context, and because of the tendency for physicians to dilate lesions seen on angiography, the angiogram is known to increase the repeat revascularisation rate (particularly for BMS) and thus potentially biases a cost-effectiveness in favour of DES on the basis that the higher the risk (as judged inappropriately in this case being the lesion itself rather than its clinical effect, the more benefit there is to be gained by reducing that risk). BASKET did not include protocol-driven angiographic follow-up and in this respect provided a sound basis for economic evaluation of DES. It is interesting to note that the non-MI related TVR rate for BMS in BASKET was 11.6% at 18 months4, not dissimilar from the 12.8% TLR seen in the TAXUS IV ‘no angiogram’ cohort at 12 months5. These data suggest that 11-13% is a reasonable estimate of 12 month repeat revascularisation rates with BMS in an unselected population where there is the rigour and reliability of follow-up associated with clinical trials but the absence of the protocol angiogram. Much of the published data both from randomised trials and registry data (Table 2) put BMS restenosis rates at about 12%-14%.
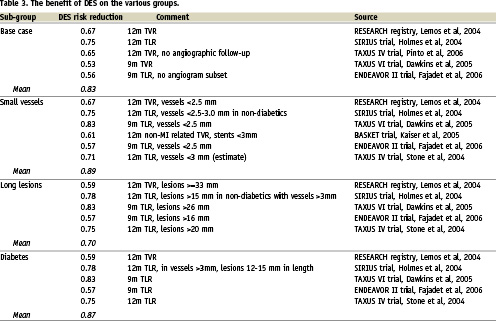
At this point, it is useful to examine the subject of time horizons. The primary endpoint of BASKET was cost-effectiveness at six months, but the continued accrual of TVR events between six and 18 months and the continued separation of the DES/BMS curves demonstrates that this time-point was inappropriate and too early to reliably estimate both the absolute rate of repeat revascularisation and the risk reduction conferred by DES4. The 18 month outcome analysis of BASKET indeed revealed that in patients receiving stents of less than 3 mm in diameter, or undergoing bypass graft PCI, DES were associated with significant reductions in death/MI, TVR and overall MACE. In these patient groups, which were largely dominated by those with small vessels, the absolute risk of repeat revascularisation was substantially higher than in the general population (about 24% at 18 months) and the risk reduction conferred by DES was also higher at about 61%. This figure of 61% fits well with our understanding of the overall risk reduction with DES (Table 3). It is notable that in the 18 month BASKET cost-effectiveness analysis, DES were more effective and less expensive then BMS in the small vessel/bypass PCI group (i.e. the dominant strategy) and a number of other high-risk sub-groups6. Other data (see below) extends the at high risk group.
However, the finding that the occurrence of the hard endpoints of death/MI was significantly reduced by DES in BASKET is not only hugely important from the patients’ point-of-view, but also from the cost-effectiveness perspective. The avoidance of these events confers long-term QALY gains that need to be modelled over longer time periods for their impact to be fully reflected in the economic evaluation7 and see above. The emerging registry mortality benefit outlined above should undoubtedly be modelled to explore the question ‘what if this effect is real’? As is clear above it can be estimated that one death avoided has the same QALY benefit as about nine TLR-PCIs avoided, thus mortality will dominate the ICER as the time horizon of the analysis extends beyond one year.
A further ‘real world’ perspective of DES economics was reported in the cost-effectiveness analysis of the Rotterdam RESEARCH registry8. Here, the approach was to use both resource-use information relating to the index procedure, and follow-up costs for repeat angiography and revascularisation, to calculate the overall costs of BMS and sirolimus-eluting stent (SES) treatment at one and two years. Results were expressed as ‘incremental cost per repeat revascularisation avoided’, in other words, ‘how much extra does it cost to avoid one repeat revascularisation by using SES rather than BMS?’. The authors concluded that SES were not cost-effective by this measure in an unselected population, but did not investigate higher-risk sub-groups. They noted that although SES were undoubtedly effective, the price premium over BMS at the time (€1,237) was too high to allows SES to be cost-effective in all patients. However, it would be interesting to see this analysis re-run incorporating 1) the absolute 3.0% mortality benefit of SES over BMS in the larger Throaxcenter cohort, reported by Serruys in evidence to the FDA advisory panel9 and 2) known utility values to calculate an incremental cost per QALY gained, including both repeat revascularisation and mortality gains. It is highly likely that a QALY-based approach would improve the cost-effectiveness of SES substantially, given the significant mortality difference. In addition, the fall in DES prices that has occurred over the past two years will also reduce the ICER. It should be noted though, that a disease-specific measure such as incremental cost per revascularisation avoided has value alongside the QALY, as the QALY may lack sensitivity to the full effect of repeat revascularisation on a patient’s quality-of-life.
In the UK, NICE have recently announced their final determination of the cost and clinical effectiveness of DES. In something of an (in the view of clinicians an appropriate) turnaround from the draft guidance, they have continued to recommend DES for use in patients with lesions longer than 15 mm or vessels less than 3 mm in diameter (but bizarrely, note not diabetics) It is apparent that a major factor in the turnabout was the advice given as clinical input by the British Cardiovascular Intervention Society (BCIS) as to what constituted the most representative and reliable data inputs to inform the economic model. Key data included the identification of a 13% risk of repeat revascularisation in an unselected population, relative risks of 1.75, 1.35 and 1.52 for small vessels, long lesions and diabetes respectively and risk reductions of 69%, 70% and 61% for those same sub-groups. BCIS’s own economic evaluation showed that at a price premium of £300-£400 over BMS, ICERs are £19,383/QALY for small vessels, £16,493 for long lesions and £18,215 for diabetes. This suggests that using the benchmark of £20,000/QALY, below which NICE consider a treatment to be cost effective based on the ICER alone, DES are cost effective in these indications at a price premium of approximately £300-£400.
Finally, decisions about the access to medical technology such as DES need to take into account the wider implications for the healthcare system and should not be based solely on the ICER – a somewhat binary measure with often disputed thresholds. BCIS have estimated that had NICE’s draft guidance prevailed, cardiologists would have had to refer large numbers of patients at high risk of restenosis back to bypass surgery because they could not be effectively treated with BMS. The net effect of this was estimated to be about a 40% increase in bypass surgery and a net cost increase to the health service of £55-£60 million. It is important therefore that heath technology assessment groups take account of the wider implications of their recommendations and do not rely solely on an economic model only considers a simple choice of one therapy/device versus another.
Cohen10 recently examined the wider impact of DES use on the costs and outcomes of revascularisation in a United States medicare population of over 30,000 patients pre- and post-DES. As well the expected reduction in repeat revascularisation, DES were also associated with relative falls in risk-adjusted mortality and MI of 12% and 29% respectively over 13.5 months median follow-up. Furthermore, overall spending on cardiovascular care fell by over $1,900 per revascularised patient.
In summary, both BASKET and Rotterdam economic studies have suggested that DES may not be cost-effective in unselected populations and this is supported by BCIS. For higher risk groups they are undoubtedly cost effective. Furthermore the emerging patterns of mortality and/or MI benefit in some large-scale studies such as the Medicare cohort now suggest that at the very least, the effect of these hard endpoints should be explored in economic evaluations as they are likely to have a significant bearing on QALY-based approaches and of course, patient outcomes.

