Abstract
Aim: Few studies are available on magnetic navigation system (MNS) for coronary artery disease. The present investigation was conducted to evaluate the feasibility and safety of guidewire navigation with a MNS in diseased coronary arteries.
Methods and results: Between February 2004 and August 2005, a total of 59 patients (68 target vessels) underwent magnetic-assisted guidewire navigation in coronary arteries with stenoses amenable to coronary intervention. Patients were excluded if any of the following conditions applied: pacemakers or cardioverter defibrillator implanted; acute myocardial infarction; thrombotic lesions; chronic total occlusion; claustrophobia; and renal dysfunction with serum creatinine > 2.5mg/dl. Procedural success was defined as successful guidewire passage distal to the stenoses with no procedural events, defined as either perforation or dissection. Magnetic guidewires successfully crossed 60 (88%) lesions. Only 1 patient experienced a minor dissection of vessel wall in a circumflex lesion and was treated with a coronary stent. There was no other complication related to the magnetic guidewire. Nineteen (27.3%) coronary interventions were performed with magnetic guidewires. In 4 complex lesions (a lesion located in saphenous vein graft with proximal severe tortuosity; 3 approaches to stent-jailed side branch), where conventional wires failed to cross the culprit lesions, magnetic navigation achieved successful guidewire passage.
Conclusions: Guidewire navigation using a MNS was feasible and safe. In the four complex cases, magnetic navigation enabled crossing of lesions in which conventional attempts failed. Further studies are needed to better evaluate the efficacy of MNS compared with conventional manual navigation.
Introduction
Devices in interventional cardiology have developed over the years to facilitate treatment of complex lesions. In particular, since the first steerable guidewires were introduced, guidewire technology has continued to remarkably improve with regard to tip softness, trackability through vessel curvatures, radiographic visibility and precise torque control. These refinements have allowed physicians to maneuver guidewires more easily. However, physicians occasionally encounter difficulties in reaching the target lesion, especially when vessel configurations are complex. There are still problematic lesion areas for manually-steerable guidewire: for instance tortuous or angulated vessels1,2 and access to the side branch through the stent strut (stent jail). Treatment of lesions in a tortuous vessel is still associated with a certain incidence of emergency coronary artery bypass surgery because of the complication or failure of PCI in these patients3. Besides, moderate and severe tortuosity significantly increases the fluoroscopic time and radiation dose in interventional procedures4.
Recently, a novel magnetic navigation system (MNS) has been introduced5,6. In an experimental study using a complex vessel phantom, magnetic-navigated guidewiring was significantly associated with shorter procedure and fluoroscopy time7. The system has already been utilized in interventional neuroradiology and cardiac electrophysiology6,8,9. In February 2004, this system was installed in our hospital and has been employed in our daily practice of coronary intervention. The present investigation was made to evaluate the feasibility and safety of guidewire navigation with a MNS in diseased coronary artery trees.
Methods
Magnetic Navigation System
The Magnetic Navigation System (Niobe™, Stereotaxis, St Louis, MO, USA) (Figure 1) and the dedicated magnetic guidewire were described elsewhere7.
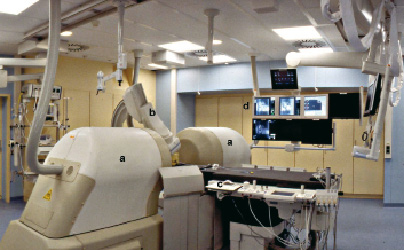
Figure 1. Photograph of magnetic navigation room. The two permanent magnets are located on opposite sides of the patient table (a). These magnets can be stored by rotating them completely out of the way of the patient table when not in use. The system is integrated with a C-arm single-planar digital angiography system (b). The operation is performed using a computer mouse or the vector tablet (c) or a touch screen (not shown). The operator can use a user interface displayed on the screen that controls the physical movement of positioners using a vector that determines the orientation of the tip (d).
Briefly, the system consists of two permanent magnets positioned on either side of the fluoroscopic table and integrated with a C-arm digital angiography system (AXIOM Artis dFC™; Siemens, Forchheim, Germany). The magnets are mounted on mechanical positioners and create a uniform magnetic field (0.08 Tesla) of approximately 15 cm inside the chest of the patient and orient the tip of the magnetic guidewire in the desired direction. The magnetically-navigable guidewire (CronusTM Floppy, Stereotaxis) has a coiled distal segment where a gold-encapsulated neodymium iron boron magnet is embedded. Reshaping of the wire tip is done only by the applying magnetic force. The mechanical deflection force at 90º that the magnetic wire can exert is 0.56 g with the application of 0.1-Tesla external magnetic field. This is lower than the push force of conventional wires7. In other words, this force is sufficient only to orientate the tip. The magnet attached at the tip is a solid 3 mm long magnet which aligns itself in the direction of the applied magnetic field when placed into the magnetic field generated by the MNS. With omni-directional distal tip control, physicians are able to reshape the guidewire tip in vivo without removing or replacing the wire. The physicians operate a computer user interface that controls the desired orientation of the magnetic field by adjusting the external magnets creating a magnetic vector that determines the orientation of the guidewire tip with the use of a touch panel. The physicians can choose a navigation strategy from four available options: (1) 2-dimensional, (2) 3-dimensional, (3) vessel navigation or (4) using presets. When the magnets are in the navigation position, rotation of the X-ray tube is limited between 30-degree left anterior oblique (LAO) and right anterior oblique (RAO). When not in use, the magnets can be rotated out of the way of the table to permit conventional procedures without any physical obstruction.
Since February 2005, this system has been integrated with a newly developed 3 dimensional (3D) quantitative coronary angiography (QCA) system (CardiOp-B™ version 1.5.xx, PAIEON, Rosh Ha’ayin, Israel)10. This system integrates information from a DICOM format created by 2 coronary angiographic images into a 3-dimensional reconstruction of an arterial segment. The 2 acquired images should have different rotation angle with at least more than 30 degrees between each other. The concept of the reconstruction is based on both edge detection algorithm and densitometric technique. Figure 2 shows a plain image of coronary angiography and its corresponding 3D reconstructed image.
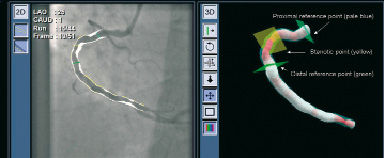
Figure 2. Coronary angiographic image (left) and corresponding 3 dimensional (3D) reconstructed image (left) of one case. The coloring of the 3D image is based on a gradient between white (healthy vessel) and dark red (99% cross-sectional area stenosis). The squares in pale blue, yellow and green denote proximal reference point, most stenotic point and distal reference point of the target lesion, respectively.
The 3D color varies according to the severity of stenosis. The coloring is based on a gradient between white (healthy vessel) and dark red (99% cross-sectional area stenosis). The system provides area and diameter data. The magnetic navigation system incorporates the 3D reconstructed image of target vessel. Physicians can align the direction of the guidewire by touching the 3D coronary path on the touch screen located beside the patient table (Figure 3).
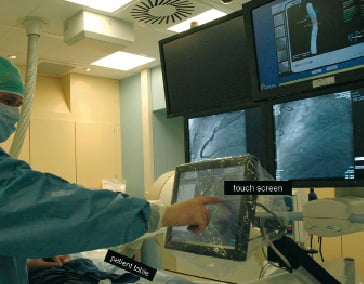
Figure 3. How does the physician operate the magnetic navigation? In the current system incorporated with 3 dimensional (3D) vessel image, the physician can align the direction of the guidewire by touching the 3D coronary path on the screen located beside the patient table. Alignment of the wire direction and advancement of the wire can be performed by one physician. The wire direction is systematically changed prior to advancement.
Alignment of the wire direction as well as advancement of the wire can be performed by one physician.
Patients
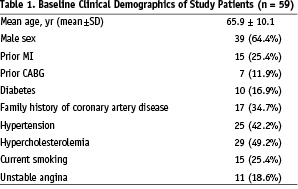
Between February, 2004 and August, 2005, a total of 59 patients with a total of 68 target vessels underwent magnetic-assisted guidewire navigation for coronary artery disease. The study protocol was approved by the local ethics committee. The study protocol was approved by the local ethics committee. Baseline clinical characteristics are shown in Table 1.
All patients had documented coronary artery disease amenable to PCI with a target lesion located in a native coronary artery or in a graft vessel. Patients were excluded if any of the following conditions applied: contraindications to exposure to strong magnetic fields (including but not limited to the presence of implanted pacemakers, implantable cardioverter defibrillators, neurostimulators, aneurysm clips, and cochlear implants); acute myocardial infarction; evidence of visible thrombus; chronic total occlusion; claustrophobia; and end-stage renal disease with serum creatinine > 2.5mg/dl. Procedural success was defined as successful guidewire passage distal to the stenosis with no procedural events, defined as vessel spasm, perforation or dissection. Written informed consent was obtained from all patients.
Angiographic analysis of target vessel/lesion
We have analyzed visual and quantitative assessment of each target vessel and lesion. All the quantitative data were obtained from the 3D QCA system.
Results
Flowchart of 68 lesions is shown in Figure 4.
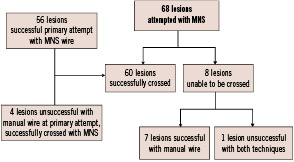
Figure 4. Flowchart of 68 lesions undergoing magnetic navigation system.
Lesion and procedural demographics are reported in Table 2.
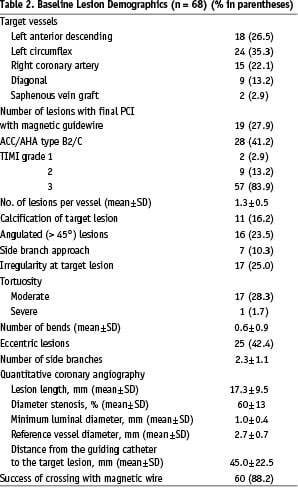
The operators used only 2-dimensional or 3-dimensional navigation among the 4 available options. Of the lesions that were attempted with MNS, 60 (88%) lesions were successfully crossed with magnetic guidewires. Because this was our first clinical experience with the application of the magnetic navigation system into coronary artery tree, we decided to initially test the navigation capability of the system in a limited manner. In 41 out of the first 60 attempted lesions, we used a magnetic wire to only cross the lesions. After having acquired some experience, we performed 19 coronary interventions using magnetic wires without exchanging for conventional guidewires (Table 2). In 56 of the 60 lesions, the magnetic guidewire was used as the primary wire. In the remaining 4 complex lesions (one lesion located in a saphenous vein graft with severe proximal tortuosity; the other 3 were side branches requiring cannulation through stent struts), conventional wires failed to cross the culprit lesions, and magnetic navigation achieved successful guidewire passage. Among the 8 cases where MNS was unsuccessful as the primary attempt, 4 cases had anatomical features of eccentric lesions located in angulated segment (Figure 5), 2 cases were due to suboptimal projection with substantial overlapping of the target lesion with other branches.
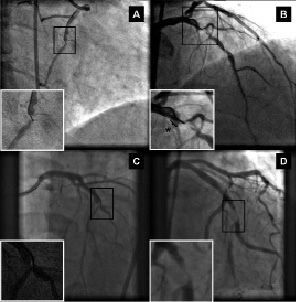
Figure 5. Angiographic images of 4 eccentric lesions located in angulated segment unsuccessful with the magnetic navigation system (overviews of target vessel with enlarged views of corresponding target lesion). Locations of the target lesions are mid right coronary artery (A), proximal left anterior descending (B), mid left anterior descending (C) and proximal obtuse marginal branch (D).
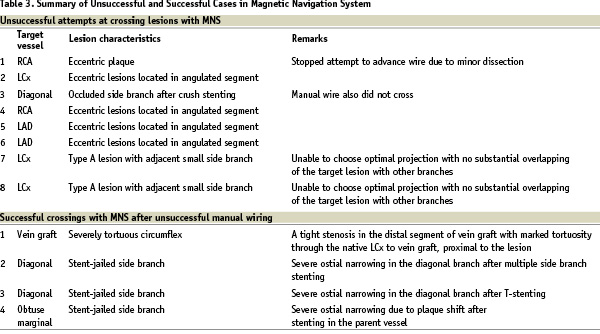
One patient experienced a minor dissection of target vessel wall and was treated with a coronary stent. Conventional manual navigation failed as a primary approach in 4 cases where magnetic navigation subsequently succeeded. PT Graphix Intermediate™ (Boston Scientific, Natick, MA, USA) conventional wires were used first in all 4 cases. Two of them (severe tortuous left circumflex and 1 stent-jailed side branch) were attempted with a conventional manual wire for more than one and half hours. After the unsuccessful attempts, these 2 cases underwent magnetic navigation at a later date. In the remaining 2 cases, conventional manual navigation was attempted for more than 10 minutes and then magnetic navigation was applied in the same session. Magnetic wires successfully crossed the lesions in less than 15 minutes. The decision to abandon manual wire navigation was entirely left to the discretion of each physician. There were no other complications related to magnetic guidewires. Finally in one case with an occluded diagonal branch immediately after crush stenting, neither MNS nor manual guidewires were able to cross. These successful and unsuccessful cases are summarized in Table 3.
Discussion
In general, when manually steering guidewires through several turns in tortuous vessels, physicians may lose torque due to the tortuosity and control of the wires because of the deformation of the preshaped wire tip. On the contrary, the tip of the magnetic guidewire can be oriented in any direction, and reshaped at any time during the procedure, irrespective of its anatomical position, location, or path crossed to get to that location. This study demonstrates the potential advantages of magnetically navigating specially designed guidewires in tortouous anatomy. Although there was only one case in this study with severe vessel tortuosity, this anatomical feature may potentially benefit from the use of MNS. Another potential advantage of MNS is for a stent-jailed side branch. When using a manual guidewire to approach stent-jailed side branch, it is recommended that the main branch wire is pulled back to the level of the most distal stent strut at the level of the side branch to cross into it in order to obtain optimal scaffolding with post balloon dilatation. However, access to the side branch through the strut of the stent may be possible through 2 or 3 different cells and can be tricky because the guidewire tip has a tendency to prolapse especially when approaching an angulated side branch. Magnetic navigation can be useful for this lesion subset as well because MNS allows physicians to alter the bend of wire tip in situ with precise direction of the wire tip using 3D navigation.
In these preliminary clinical experiences, it was suggested that the following 2 factors may be associated with unsuccessful magnetic navigation: 1. eccentric lesions located in angulated segments; 2. limited X-ray projection angle which might cause suboptimal angiographic projection. Improvements are underway to overcome these 2 issues. Modifications to shorten the length of the magnet attached to the guidewire tip have been made. Guidewires with a short segmented magnetic tip would be one possible solution in such short angulated lesions. A new wire with short magnetic (2 mm) tip has been developed and currently evaluated for clinical use. An advanced model of the external magnet system can permit up to 50 degrees of C-arm articulation by allowing the magnets to arc along with the x-ray system (Figure 6).
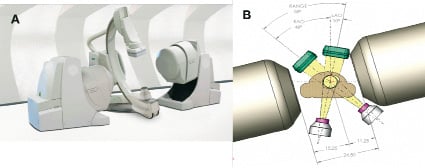
Figure 6. New magnet system (Niobe™ II, Stereotaxis). Illustration A represents the overview. This retractable and smaller magnets compared to the current magnetic positioner allow more extended (more than ±15º) rotation angle of image intensifier (B).
Greater angulation (additional ±15º rotation angle compared to current magnetic positioner) of the image intensifier enables physicians to choose more extreme projections to remove any overlap of the target lesion with other branches.
Study limitation
Study population was small and this paper discussed only descriptive analyses.
In terms of successful guidewire navigation, chronic total occlusions are the ultimately challenging lesions. These lesions were excluded from this study.
Conclusions
This study describes the clinical experience of magnetically-navigated guidewire use in human coronary artery stenoses. Guidewire navigation using a MNS was feasible and safe. The four sporadic cases involving tortuosity in which a manually steered guidewire failed at the primary instance suggest that MNS may be useful for approaching more complex coronary lesions. At the same time, some limitations of the current system and the magnetic guidewire architecture also became apparent. Additional improvements are anticipated to facilitate magnetic guidewire passage in the lesions that the current system failed to cross.
Acknowledgement
The authors wish to thank Hans Meulenbrug, BSc (Stereotaxis Inc.) for his technical support of the system.
Dr. García-García is supported by Sociedad Mexicana de Cardiología.

