In this issue of the journal, Dr. Serruys and colleagues present data from a non-inferiority trial comparing the paclitaxel eluting stent (Taxus Express) to the everolimus eluting coronary stent (Xience V)1. In this relatively non-complex group of patients and lesions (i.e. reference vessel diameter 2.7 mm with average lesion length 13 mm), the Xience V stent outperformed the Taxus stent in terms of the primary endpoint of in-stent late loss. These findings suggest that, with respect to restenosis prevention, the Xience V stent may be a formidable newcomer to the DES market.
The excellent results produced by this DES system can best be understood by examining its three major elements: the bare metal stent backbone, polymer coating, and the drug with its release kinetics. Impressive reductions in neointimal formation can only be achieved when arterial injury is minimised, polymer biocompatibility is maximised, and the choice of drug and drug release kinetics are appropriate.
Stent strut thickness plays a significant role in neointimal formation. Randomised trials have demonstrated that bare metal stents (BMS) with thin struts have significantly reduced restenosis rates when compared to thick-strut BMS, presumably because stents with thinner struts cause less arterial injury2-4. The Vision stent, the bare metal backbone of the Xience V DES, has a strut thicknes of 81 µM, which compares favourably to the 132 µM Express stent (Taxus stent backbone). Consequently, DES with thinner struts may result in less neointimal growth by reducing activation of pathways that lead to proliferation and migration of SMCs.
The currently used non-erodable polymer coatings employed in Cypher and Taxus stents are known to provoke significant inflammatory responses that can themselves increase neointima and perhaps interfere with arterial healing5,6. We have shown both in animal models and in humans that Cypher and Taxus DES induce distinctive inflammatory responses that persist for a long period of time7,8. Importantly, the relationship of lack of polymer biocompatibility to neointima formation and delayed re-endothelialisation remains unknown.
Drug choice and release kinetics are probably the most important components of DES technology, especially as they relate to the vascular responses elicited by these devices. A considerable amount of data exists on how sirolimus (and its analogue, everolimus) and paclitaxel are different in terms of their effects on the arterial wall. While both reduce restenosis by disrupting smooth muscle cell cycle progression, their modes of action differ. Everolimus inhibits the mammalian target of rapamycin (mTOR) and prevents the degradation of p27kip, a cyclin-dependent kinase inhibitor that plays an important role in regulating vascular smooth muscle cell migration and proliferation9.
In animal models, reduction of neointimal growth by rapamycin results in persistent fibrin deposition and delayed arterial healing compared with BMS10. Oral everolimus was previously shown to dose-dependently suppress neointimal formation in a rabbit model of iliac artery stent implantation11. High-dose everolimus treatment resulted in markedly reduced neointimal formation, which coincided with delayed arterial healing, characterised by prolonged fibrin deposition and poor re-endothelialisation. While such signs of delayed arterial healing were absent in animals treated with lower dosages of everolimus, a similar benefit in terms of neointimal inhibition was observed in this treatment group. These findings suggest a wide therapeutic index of everolimus, thus establishing the basis to balance these two ambitious efforts.
Paclitaxel acts predominately during the mitosis (M) phase of the cell cycle through suppression of microtubule spindle formation needed for cell replication, and is known to induce cytotoxic effects either by induction of apoptosis or necrosis12. While both, everolimus and paclitaxel inhibit proliferation of SMCs and endothelial cells (ECs), only paclitaxel disrupts migration of ECs at therapeutic concentrations13. Heldman reported increased medial wall necrosis, smooth muscle cell loss, and arterial dilation with increasing stent based doses of paclitaxel14. Taxus stents implanted in an overlapping fashion in the rabbit model, which resulted in a higher drug dose at the overlapping site, produced dose-dependent reductions in endothelialisation, increased fibrin deposition, and caused significant arterial wall dilatation and medial cell loss. These findings are consistent with arterial toxicity and indicate a narrow therapeutic index for this drug, even when delivered locally. This toxic effect may be amplified by the biphasic drug release profile, characterised by an initial burst of drug, followed by a constant slow release, which continues to elute the drug even after 180 days7. It is important to emphasise that appropriate drug choice, along with optimised release kinetics are fundamental determinants affecting the long-term success of current and future DES platforms.
In aggregate, these data help to explain the superior results of the Xience V DES. However, these findings should be considered preliminary. Declaration of the superiority of the everolimus eluting stent over existing polymer based DES awaits further data from more complex patient subsets, which include small vessel sizes, longer stents, and more complex lesions as well as patients (i.e. higher proportion of diabetics). Moreover, recent concerns about long-term delay of arterial healing produced by both the Cypher and Taxus DES7 and the attendant risk of late stent thrombosis, means that trials powered for late loss (such as this one), with relatively few patients and short term follow-up cannot establish the long term safety of this DES system. Appropriately, this trial incorporates 2 year repeat angiograms and IVUS follow-up, which may give us more information about the healing characteristics of Xience V.
Nonetheless, the preclinical data for the Xience V stent are encouraging in terms of arterial healing. We recently examined the rates of re-endothelialisation in Xience V and Taxus Express DES in two separate studies utilising the porcine model of coronary stenting. Endothelialisation was evaluated by scanning electron microscopy at 14 days. Xience DES had superior endothelialisation compared to Taxus DES, with greater strut coverage and better endothelial cell junctions (Figure 1).
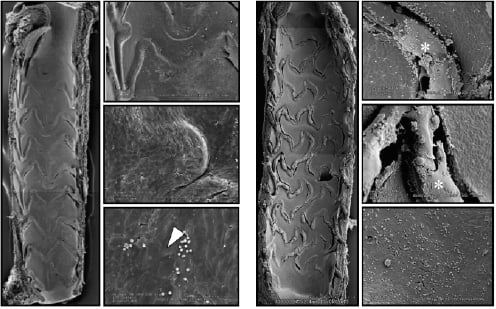
Figure 1. Representative scanning electron microscopy images from Xience and Taxus drug eluting stents in low and high power magnification. Xience DES (A) show almost complete re-endothelialisation, with tight intercellular junctions (arrowhead), while there is incomplete strut coverage in Taxus DES, along with persistent fibrin deposition (*).
Although this suggests a good safety profile of Xience DES at short-term follow-up in pre-clinical studies, as previously mentioned, long term data in humans are needed to confirm the significance of these findings.
In conclusion, the results of the SPIRIT II trial are encouraging and represent an advance in terms of prevention of restenosis in this relatively non-complex series of patients. The real test of this stent, however, is not whether it can equal or better its rivals in terms of late loss, but whether its long term safety record is superior to its counterparts. From the standpoint of its design and the emerging preclinical data presented here, it may surpass its rivals but this awaits more long-term data in humans.

