Introduction
Within the cardiology community there continues to be confusion regarding the indications for percutaneous coronary interventions (PCI) in patients with chronic total occlusion (CTO) and scepticism with regard to the ultimate impact revascularisation has on patient outcomes. It is not surprising, therefore, that most interventional cardiologists try to avoid these potentially long procedures that can be costly and expose the operator to higher radiation doses1,2 and, with success rates remained unchanged in the last years, perceived as insufficient to justify the effort involved. The procedural complexity of CTO angioplasty and the lack of familiarity with new equipment and techniques often prompts half-hearted and prematurely aborted attempts at PCI, leading to physician and patient frustration. A recent analysis from a NHLBI database even showed a decrease of attempted PCIs in CTOs from 9.4 to 5.7% between 1997 to 20043. Consequently, patients with single vessel disease and chronically occluded vessels are often managed medically regardless of the severity of symptoms and extent of ischaemia, and those with multivessel disease with a CTO are referred for bypass graft surgery even if the other lesions are ideal suited for PCI4-7. As a reaction to this prevailing attitude, experienced European interventionists have recently established the EuroCTO Club, modelled on similar initiatives in Japan and the USA, whereby members share their experience both within the group and with the interventional and general cardiology communities at large.
The aim of the EuroCTO Club is to promote clinical excellence through training and quality control as well as fostering research and technical development in the field of CTO recanalisation. The current paper is an essential part of this strategy: we do not wish to challenge or overcome other recent comprehensive reviews of this broad subject8-12, but rather, our aim is to highlight misconceptions in clinical indication, outdated technical choices, inadequacies in operator training and centre qualifications and equipment which limit a more widespread application of percutaneous techniques for recanalisation of CTO in Europe and impair the consistent achievement of the high success rates possible with contemporary techniques.
Definitions and clinical indications
CTO lesions: definitions and characterisation into subgroups
There appears in the literature a number of ways of defining a CTO, with some authors including lesions with distal TIMI 1 flow and occlusion of 1-3 month duration. The consensus among the group is to define a CTO as “the presence of TIMI 0 flow within the occluded segment with an estimated occlusion duration of > 3 months”.
TIMI 0 FLOW
The identification of TIMI 0 flow is not as straightforward as in recent post-MI occlusions, for which the TIMI classification was originally developed. Antegrade contrast filling of the segment beyond the occlusion does not preclude TIMI 0 flow within the occluded segment. Non-intra-luminal ipsilateral bridging collaterals may give antegrade flow and the false impression of a functional incomplete occlusion. Their presence should be differentiated from TIMI 0 flow within the occluded segment by careful frame-by-frame assessment in different angiographic planes13,14.
The presence of intraluminal channels certainly plays a role in facilitating crossing of occlusions. Pathology shows that they are often below the threshold of angiographic resolution13,15. Antegrade contrast filling of the segment beyond the occlusion flow, in the absence of ipsilateral bridging collaterals and even when the occluded vessel segment shows no intraluminal contrast filling, indicates a functional and not a true CTO as defined above. Only with meticulous filming with a vigorous contrast injection with a well engaged catheter allows us to conclude that TIMI flow is 0 within the occluded segment and the lesion should then be classified as a CTO if it corresponds to the occlusion duration criteria listed below.
OCCLUSION DURATION
Since the time of occlusion is not always known, we suggest 3 levels of certainty:
a) Certain (angiographically confirmed): the minority of cases where a previous angiogram (for instance before a previous CABG operation, or after an acute myocardial infarction) has confirmed the presence of TIMI 0 flow for > 3 months prior to the planned procedure;
b) Likely (clinically confirmed): objective evidence of an acute myocardial infarction in the territory of the occluded artery without other possible culprit arteries > 3 months before the current angiogram;
c) Possible (undetermined): a CTO with TIMI 0 flow and angiographic anatomy suggestive of long-standing occlusion (collateral development, no contrast staining) with stable anginal symptoms unchanged in the last 3 months or evidence of silent ischaemia; in case of recent acute ischaemic episodes (acute myocardial infarction or unstable angina or worsening effort angina), a culprit artery other than the occluded vessel should be present.
CONSENSUS ON DEFINITION
Lesions can be classified as CTOs when there is TIMI 0 flow within the occluded segment and angiographic or clinical evidence or high likelihood of an occlusion duration > 3 months.
Clinical indications
As with all stable lesions in ischaemic heart disease, the aim of revascularisation in CTOs is to improve symptoms and/or prognosis16,17. Reopening of a CTO should be considered in the presence of symptoms or objective evidence of viability/ischaemia in the territory of the occluded artery. Several studies have documented that successful treatment of CTO leads to an improvement in anginal status, normalisation of functional tests, improvement of left ventricular function and avoidance of CABG18-27. In addition, there are data from large retrospective studies showing that patients with a successful recanalisation of a CTO have a lower mortality and need for CABG treatment than patients with unsuccessful procedures28-30. Patients with untreated CTOs face a threefold increase in cardiac mortality or complications in case of future acute events31,32.
As evident from the definition (> 3 month occlusion duration), the indications to revascularise a CTO are not affected by the heated debate on the need to ensure patency of all infarct-related arteries. The recently published Open Artery Trial (OAT)33,34, as well as other trials on this topic35,36, typically have among their inclusion criteria an interval < 30 days after acute MI and evidence of viability and/or ischaemia was not required for enrolment. Only half of all patients with CTOs have Q-waves or akinesia in the territory of the occluded artery27,37. In those patients with evidence of previous MI and impaired regional LV function – but ideally in all CTO patients – a thorough non-invasive investigation before treatment is highly recommended to establish the presence of ischaemia and viability in the territory of the occluded artery. Therefore, it is inappropriate to apply the conclusions of the OAT trial to CTOs, being completely different clinical scenarios.
CLINICAL PRESENTATION AND TREATMENT STRATEGY
The clinical presentation of a CTO can be very variable. On one hand there are patients with stable angina, silent ischaemia or heart failure of ischaemic origin, while on the other hand we see patients with new-onset angina or undergoing primary PCI due to acute occlusion in a different culprit vessel, and in whom the CTO is discovered as an incidental finding.
The first group provides the greatest challenge to decision making, especially in multivessel disease where there may be a natural tendency to refer to surgery rather than embarking on a multivessel PCI, knowing that with the CTO present it could possibly lead to an incomplete revascularisation30. The presence of a CTO, however, should not be sufficient reason to deny percutaneous revascularisation in the absence of significant left main disease, and when the other lesions are suitable for PCI, especially, if there are relative or absolute contraindications to surgery. For an individualised treatment strategy, the identification of predictors of crossing success (see below) are helpful, and in controversial cases, the review in a Cardiology-Cardiac Surgery meeting or sharing opinions among experienced interventionists is strongly recommended.
If the decision is taken to undertake PCI, a staged approach is often a reasonable strategy in multivessel disease in order to avoid excessively long procedures and large amount of contrast media. Consideration of which artery to tackle first, the CTO or the non occluded vessel(s), should be based on the importance of the occluded vessel (if the vessel and the amount of viable myocardium is important, the CTO should be approached first, while with poor contralateral flow or intended retrograde approach the stenosis in the contralateral vessel may need to be treated first). Additionally, inverted collateral flow through the recanalised CTO may protect myocardium at risk during treatment of high risk complex lesions in the collateral donor vessel. It is important that each case is considered individually and carefully and that the consequences of success or failure of the individual lesion treatments be taken into account.
In acute coronary syndromes, the use of a staged procedure with immediate initial treatment limited to the culprit artery is often easier and clinically sound. There is no doubt that ST-elevation myocardial infarction treatment should be limited to the culprit infarct related vessel and that all other lesions – especially CTOs – should be referred for subsequent evaluation and possible treatment depending on evidence of ischaemia and viability. Furthermore patients with acute coronary syndromes often need rapid recanalisation of the culprit vessel, in the presence of glycoprotein IIb-IIIa inhibitors or bivalirudin administered prior or during the procedure. CTOs should not be attempted with these pharmacologic agents on-board, and therefore a staged approach is a safer choice.
Not infrequently, with both acute and chronic presentations, the CTO is left untreated after one or more non-occluded arteries have been stented. This is often caused by miscommunication between the interventionalist doing the initial angioplasty and the referring cardiologists. Even if the patient becomes asymptomatic or his symptoms are acceptably controlled by medical treatment, it is important that a sensitive non-invasive provocative testing is performed to rule out the presence of large areas of residual and possibly silent ischaemia. Angiographically visible collaterals are
often used as a justification for a conservative approach. If present before the occlusion develops, collateral filling provides sufficient blood flow to maintain viability of the myocardium supplied by the occluded artery, but is almost always insufficient to avoid the presence of effort induced angina/ischaemia. In a systematic analysis of the flow and pressure provided by collaterals to the area distal to the occluded artery, patients with CTOs will achieve only about 40% of the pressure level of an open artery38. During exercise, only about 5 percent of patients will have collaterals with enough functional reserve to increase collateral perfusion, whereas more than one-third of patients will experience coronary steal39,40. In general,
collaterals are able to maintain baseline myocardial blood supply, but they are limited in their functional reserve and will not prevent exercise induced ischaemia41. It is important that there is a careful evaluation of the clinical and imaging findings, also taking into account the potential life expectancy of the patient, which may be viewed differently in a 60 year old, as opposed to an 80 year old, patient. An overall assessment of the patient, including the clinical history, is essential to avoid younger patients having to limit their activities, and potentially suffering the side effects of anti-anginal treatment, merely because an experienced interventionist has not been involved in assessing the likelihood of success of a complex CTO. Not infrequently such patients languish around having been turned down for single-vessel bypass surgery, perceived as having an unfavourable risk/benefit balance for a treatment limited to (usually) a right coronary artery or left circumflex artery.
Despite careful clinical and functional evaluation, treating a CTO in the presence of heart failure with equivocal evidence of viability of the myocardium can be a difficult clinical decision. Radionuclide techniques and dobutamine stress echocardiography perform a relatively similar role regarding positive and negative predictive values for predicting improvements in regional function42. Magnetic resonance coupled with gadolinium injection and dobutamine/ adenosine infusion has emerged as a non-invasive imaging approach and has the potential for being an objective evaluation of pharmacologically induced wall motion changes with the early and late phases of gadolinium distribution providing a precise quantitation of myocardial fibrosis and perfusion43. If any reasonable doubt remains concerning the presence of residual viability in the territory of the occluded artery, an attempt at percutaneous recanalisation is probably still justified considering the limited risk and great potential benefit27,44.
CONSENSUS ON INDICATIONS AND STRATEGY
1. Consideration of the best treatment modalities for CTOs should be the result of a careful review of clinical history, the results of sensitive provocative tests, coronary anatomy, and personal experience.
2. With average recanalisation success rates of > 70% in experienced hands using contemporary CTO techniques, the presence of a CTO should not be a sufficient reason to switch from a percutaneous towards a surgical approach in multivessel disease.
3. In general ad hoc angioplasty is not recommended in the presence of CTOs which need proper procedures planning.
Operator experience and centre requirements
Training in CTO recanalisation
In Europe, specific training in interventional cardiology is not required and most new specialists begin interventional cardiology upon completion of their training with limited theoretical knowledge and often only modest practical experience. To correct this anomaly, the Educational Committee of the European Society of Cardiology has ratified, in the recent 2006 Core Curriculum for the General Cardiologist, that a level III competence (independent performance of procedures) is required for diagnostic cardiac catheterisation and coronary angiography, with a minimum number of 300 cases performed during training (http://www.escardio.org/knowledge/education/ coresyllabus). A practical experience of only 100 cases of percutaneous interventions during training is limited to level II (assistance under the guidance of a superior) and is not designed to train the candidate to be an independent operator. The need for non-interventionists to perform angiography is under debate, and may soon change with the expansion of noninvasive techniques of coronary angiography such as multislice computed tomography (MSCT). Currently, it is still important, however, that all angiographers understand that occlusions require acquisitions in multiple views, that the acquisition must be prolonged to visualise the distal segments filled by collaterals, and that the source of collaterals from less usual vessels must be optimally and selectively engaged (for example the conus branch for LAD occlusions, LIMA for RCA occlusions)45,46.
The WG 10 on Interventional Cardiology (now the European Association of Percutaneous Cardiovascular Interventions, EAPCI) published in 2005 a Curriculum and Syllabus to establish an optimal and homogeneous pattern of training in Europe47. After a 2 year training period the candidate is expected to tackle complex angioplasty as a primary operator, and CTOs are mentioned as part of the experience required. We believe that all centres involved in training of interventional cardiologists should be engaged in a regular program of CTO recanalisation. The growth in frequency and the success rate in treating CTOs in Europe is critically dependent on a robust initial process of training offered to all Interventional Fellows. Training should provide the theoretical knowledge for appropriate patient and lesion selection as well as the practical experience to avoid, at the very least, the most common mistakes made by beginners unaware of the specific technical requirements of CTO recanalisation. The training experience should be sufficient to overcome the steepest initial phase of the learning curve, allowing the trainee to comfortably approach at least the simplest CTOs with the appropriate equipment and strategy to achieve success, and to have gained sufficient knowledge and experience to stop before complications occur or, in the worst scenario, to treat efficiently the most common, specific problems.
We empirically propose that 30 CTO procedures (primary/secondary operator) are part of the 200 angioplasty package required to successfully complete training. The role of the trainee can vary, based on the complexity of the CTO procedures performed, and the level of training reached. It makes no sense that a trainee with less than 6 month experience in angioplasty, still experiencing problems in crossing simple subtotal stenoses should face the subtleties of wire handling in difficult total occlusions, but they will certainly benefit from a role as secondary operators in these complex CTO cases. For each trainee, a complete log-book should indicate in detail the CTO anatomies and techniques the candidate has been exposed to, and the supervisor should give a specific evaluation of the level of training reached in CTO recanalisation.
Operator and centre requirements
It is our general belief that newly trained operators, or those operators with low volume of angioplasties/year, should not embark on treating complex CTOs without the advice and support of an experienced operator. If this is not possible, the procedure should be rescheduled for a day when this support is available, or the patient should be referred to a centre with expertise in CTO treatment. The “chronic” nature of the pathology, its relative low number and the frequent use of staged procedures in CTO recanalisation allows for the application of this strategy with neither patient risk, increased discomfort, nor disruption of the cath lab/hospital schedule.
No legislation prevents small volume centres or PCI operators from treating complex CTOs in Japan. However a practice pattern which sees most of these procedures performed or directly supervised by “super-specialists” has become widely accepted, and this attitude is one of the underlying reasons for the extraordinary development of this technique in Japan, and of the exceptionally high success rates reported. Unfortunately, cross referral of patients with complex or very complex CTOs, or even of patients following failed CTO procedures, to centres/operators with greater experience is rare and these patients are often left on medical therapy or referred for surgery. Auditing the results of CTO treatment in each centre, and by operator, can offer an indication as to whether sufficient skills and experience have been applied. If an appropriately complex CTO case-range has been treated and the success rate of CTO does not exceed 50%, or is anyway significantly lower than benchmark figures, the operator/centre justification for continuing their program of CTO recanalisation should be reconsidered. The availability of high quality digital image intensifiers, a sufficient variety of guiding catheters and wires (including dedicated wires) and the possibility to use multiple balloons and drug eluting stents to cover the entire occluded segment are required for centres willing to maintain an active CTO program. Biplane imaging, availability of IVUS and Rotablator® are welcome additions, but cannot be considered indispensable for CTO recanalisation
If we assume that less than 10-15% of the total PCIs attempted are CTOs, and we recommend a minimum number of 50 CTO cases per year to maintain competency, a large volume laboratory with more than 1,000 cases/year can provide continuous training to no more than 2-3 operators. The current trend to allow low volume centres to start an interventional programme to reduce in-hospital waiting time and to allow local access of patient for acute procedures such as primary angioplasty, often creates centres where a workload and patient mix exists which allows no operator the possibility of performing sufficient numbers of CTO procedures to maintain acceptable competency. Transferring the patient to a larger centre, or developing a program of proctorship with guest operators coming to help for the most complex cases, are possible solutions. Absence of surgical back-up is not, per se, a contraindication to develop a CTO treatment programme, but the appropriateness of indications must be confirmed by the regular involvement of cardiac surgeons as, and when, required. The centre must also be able to confirm it has the ability to promptly deal with complications such as cardiac tamponade, as well the safe and rapid transfer of the few cases potential requiring emergency cardiac surgery48.
Radiation safety in CTO treatment
Treating CTOs exposes the patient and the operator to higher doses of radiation than most non-CTO procedures. Furthermore, even if procedures involving multivessel recanalisation of complex lesions have similar fluoroscopy times and doses, the treatment of CTOs often involves the X-ray gantry being maintained in one working view, with occasional rotation in an orthogonal and equally fixed view. The same skin and body segments are therefore exposed to the majority of the radiation dose. Even if reset to a higher level, it is important to follow the principle for CTO as per that of other procedures that the operator and patient dose remains the same, “As Low As Reasonably Achievable” (ALARA).
The following recommendations are very important to optimise radiation exposure for both the operator and patient during CTO procedures.
1. The personnel will receive radiation from 2 sources, the patient (scattering) and the X-ray tube (leakage and scattering). Especially in laboratories where CTO procedures are frequent, X-ray tubes should be extra-shielded. Using the same gantry settings, additional shielding between the patient and the operator will lower the personnel dose by a factor of 4 to 10.
4. The use of modern equipment (high output X-ray tubes) with pulsed fluoroscopy and extra beam filtering should be mandated. These filters can lower the patient entrance dose levels, while they block a great part of the non-image forming photons which are only absorbed in the patient.
5. Low dose settings, 110 kilovolt (kV) during fluoroscopy, should initially be chosen, and only if the image quality is insufficient, during the time of wire crossing (when optimal image quality is imperative), should one select higher dose settings (standard setting is 125 kV). By applying this model the scattered radiation levels are less, especially when large or obese patients are treated. One may experience more quantum noise during fluoroscopy, but a noisy image with good contrast levels could be less annoying to the eye than a grey image with less noise, while remaining equally informative.
6. For angiographic documentation, complementary to the standard digital cine mode (DCM) storage, the pulsed fluoroscopic mode (PFM) can be used which can reduce radiation exposure by a factor 4. The use of low pulse frequencies (< 15 pulses per second instead of the normal 25-30) during PFM and DCM will further reduce radiation exposure. It can be applied when treating CTOs of the left or proximal coronary arteries where movement of the vessels and guidewires is not rapid.
7. Extra collimation should also be regularly used; the beam size should be no larger than needed and should be meticulously corrected as most of the units automatically give the largest field possible.
8. Using biplane systems has potential advantages, but the dose levels are doubled compared to monoplane units. In particular, biplane acquisition is not required for the final phases of balloon dilatation and stent implantation. Also, the use of extreme caudal projections imposes high scattered doses to the operators and should be avoided unless indispensable for optimal visualisation of the occlusion stump and of the distal re-entry.
9. Radiation monitoring is of paramount importance, especially since repeat procedures are not unusual when treating CTOs. An X-ray report with the radiation used and the given patient dose must be created. In the unlikely event the dose received is so high as to be associated with a risk of erythaema, the patient should be warned and appropriate follow-up arranged.
If all these recommendations are used, patient harm will be minimised, even with 2-4 hours fluoroscopy time per procedure, and the annual investigator radiation dose can be kept at a level that hundreds of procedures per year, including numerous CTOs, can be performed without exceeding the maximal personnel dose (20 milli Sievert) recorded on the outside of the apron.
Consensus on training and centre/operator competency
1. All the interventional cardiology trainees should acquire the theoretical knowledge to be able to select appropriately candidates for CTO treatment as well as having the practical experience to avoid at least the most common mistakes in CTO recanalisation.
2. Even after the recommended 2 years of training, sufficient to work as an independent primary operator, a sensible operator should refrain from engaging in the recanalisation of complex CTOs without appropriate supervision.
3. The minimal number of 50 CTOs per year to maintain competency translates into a model where only a limited number of operators and centres should be allowed to perform CTO treatment, with selection based on workload and audited success and complication rates.
Current status of CTO recanalisation
According to data from the 1997-1999 National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry, the prevalence of CTOs increases with advancing patient age49. The proportion of patients undergoing PCI for CTO was 15.6% in the 1997-1998 NHLBI Dynamic Registry report50. while the National Cardiovascular Registry of the American College of Cardiology reported that angioplasty for CTO was attempted in only 12% of 100,292 patients undergoing PCI in 139 US hospitals from January 1998 through September 200051. It is noteworthy that the definition of CTO in these registries was, by far, less strict than the definition we have adopted above, so that the true figures of CTO attempts are likely smaller, and certainly less than 10% of all PCI procedures. Only limited data are available about incidence and treatment of CTO in Europe. The 2005 EuroHeart Survey on PCI has enrolled 13,152 patients in 134 centres and 39 European countries52. The presence of 61% academic centres and 65% centres with on-site surgery with an average annual workload per centre of 600 PCIs means that large volume centres are well represented. Of the 5,067 patients treated for stable angina/silent ischaemia, a CTO was treated in 1,606 patients, 28.6% of these elective cases and 12.2% of all the patients consecutively entered in the period June 2005-January 2006. It is difficult to ascertain how many of the lesions identified as CTO in the Survey qualified for the more stringent criteria of the definition we proposed (treatment of a lesion scored as 100% occluded in stable syndromes). Future surveys and studies should adopt this common definition to allow proper comparison of results. The 15 centres of the founding members of the EuroCTO Club attempted a CTO in 12% of the 28,243 patients receiving PCI in 2006 (range 7.8-17.5%). The personal first operator experience in 2006 of the Club members included 107 CTO cases/year (range 50-180) with a success rate of 75.1% (range 62-85%).
Technique of CTO recanalisation
Results and complications
The technique of PCI in chronic occlusions has changed dramatically in the last 15 years due to increased operator experience and the development of improved, dedicated equipment. Even so, the overall success rates are still far from the success rates of other elective PCIs and, at first glance, appear paradoxically not too different from the success rates of those series reported 10-15 years ago. The explanation of this paradox is that CTOs with complex morphology and long occlusion duration that were considered untreatable before the mid-nineties are now more regularly attempted using dedicated wires. Similarly, the main difference between experienced, dedicated and non-dedicated CTO operators is not so much in their success rate but in the frequency and complexity of the CTO lesions attempted.
The success rate decreases with parameters such as occlusion duration, length and amount of calcification. Table 1 reports a list of lesion and patient-based parameters expected by consensus of the group to be predictive of procedural success.
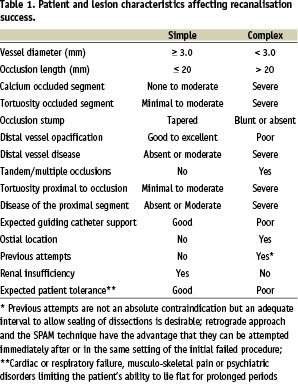
A predominance of favourable characteristics can lead highly experienced CTO-operators to a success rate > 90% while this may drop to < 60-70% in the presence of one or more unfavourable predictive factors. The EuroCTO Club is developing a European CTO database to prospectively identify predictors of procedural success and determine their relative importance.
Complications during angioplasty of chronic total occlusions
PCI of a CTO is traditionally considered a low-risk procedure, but in-hospital major adverse events occurring in excess of 5% have been reported10,21,53,54. Specific risks of CTO procedures include: acute myocardial infarction due to compromise of collateral flow; coronary perforation with pericardial blood extravasation and cardiac tamponade; damage of the proximal occluded segment due to deep intubation or selection of aggressive guiding catheters; contra-lateral vessel damage (mechanical or thrombotic) during cross; dual injection; renal failure due to excessive contrast load. In a series of 2,007 angioplasties for CTO performed over a 20 year period in a centre with extensive operator experience, peri-procedural myocardial infarction occurred in >2% of cases, emergency bypass surgery in 0.7%, and death in 1.3 % of patients28.
The Club’s experience with the 3,403 CTO patients performed in their institutions during the year 2006, appears more favourable (death 0.12%, Q-MI 0.14%, any MI 1.96%, emergency CABG 0.27% and cardiac tamponade 0.64%). This data compares favourably with the results of the EuroHeart Survey on PCI 2005, which reported a death rate and incidence of myocardial infarction in elective procedures of 0.3 and 1.1%. Only the incidence of cardiac tamponade was higher in the CTO cases of the EuroClub Investigators (0.64% v 0.1 in the EuroHeart Survey 2005.) Despite the fact that wire exit is the rule rather than the exception in crossing CTOs, major blood extravasation is unlikely when the wire becomes extraluminal through an occluded segment. In case of successful subsequent crossing, the puncture will be sealed by the dilatation of the true lumen and stent implantation. The problem normally is initiated when the operator fails to realise that the wire is not intraluminal and advances a balloon or, worse, dilates a balloon. Even so, prolonged balloon occlusion proximally and reversal of heparin with protamine sulphate is normally sufficient to stop the leakage. Because of this rare but potential problem, none of the EuroCTO Club members use anticoagulants which cannot be reverted. Agents such as bivalirudine, abciximab or other GP IIb-IIIa inhibitors are used sparingly and only after successful crossing and distal flow restoration.
Crossing the occlusion
The most common reason for PCI failure in CTOs, is the inability to pass a guidewire across the lesion into the distal vessel. There are several basic rules of successful wire steering and crossing. A careful study of the diagnostic angiography with a good visualisation of the collateral supply is required to select the appropriate guiding catheter, define the need for contralateral injection, select the guidewire, and plan the subsequent steps after wire crossing, such as the anticipated support needed for balloon and stent advancement. It is crucial to carefully review all cine runs in order to select the optimal working projections, at least 2, ideally 90 degrees apart. In each, the operator must select the position where the stump needs to be punctured, draw an imaginary line across the occluded segment and detect the location and direction of the distal re-entry.
ACCESS ROUTE
The selection of the access route is dependent on the individual patient situation – e.g. severe peripheral vascular disease, which may mandate a radial approach – as well as on the operator preference. Guiding catheter size is limited from the radial approach, but the radial artery can be easily used for contralateral injection (5 or 6 Fr diagnostic catheters).
GUIDING CATHETER SELECTION
The guiding catheter selection is the first key to success. It is important to provide maximum support for wire crossing and subsequent balloon advancement, using coaxial orientation, good stability and optimal back-up force of the guiding catheter. The catheter should withstand prolonged exposure to blood at 37 degrees without rapid deterioration of its handling characteristics. Either a large lumen guide of 7Fr/8Fr guide catheters offering greater passive support, or a large lumen 6 Fr/5 Fr guide with good active support after deep insertion into the proximal segment of the target artery are options. There is a somewhat discordant practice among the CTO members with general consensus that the use of 6 Fr catheters is probably sufficient for more straightforward CTOs while in the most complex lesions only 7 or 8 Fr guiding catheters are sufficiently large to advance 2 wires and 2 OTW catheters (for parallel wire technique). Even with modern 6 Fr guides (0.70-0.71” inner lumen) only 2 Monorail catheters of some manufacturers with thin walls can be used. If an IVUS guided technique is used, 8 Fr guides are required to advance an IVUS catheter side-by-side with an OTW catheter. When selecting the guiding catheter size, it should also be kept in mind that the usage of contrast media will be lower with 6 Fr catheters, than with large 7 or 8 Fr catheters.
The choice of guiding catheter shapes is also dictated by personal experience and preference. For the right coronary artery (RCA) most operators will start with Judkins right (JR) catheters to avoid the risk of ostial damage, and use deep intubation or an anchor technique to obtain more support if required. Also in cases where there is very proximal occlusion of the RCA, a JR is felt to be the best option, especially in 7 or 8 Fr to provide greater support. Others would select routinely Amplatz Left (AL) 1 or 0.75, preferably with side holes in case of inadvertent deep intubation and small arterial diameters. For the so-called “shepard’s crook” proximal RCAs an AL 1 or 2 may represent the best choice. For CTOs of the left coronary artery, modern extra backup shapes (e.g. XB, EBU, Voda) are preferred to classical Judkins left catheters. For the left circumflex artery (LCx), especially in the presence of a short left main, an AL 2 or 3 may provide better support and coaxial orientation.
CONTRALATERAL INJECTION
When the distal vessel is mainly filled by retrograde collaterals, or there are bridging collaterals originating near the occlusion that are likely to have their flow impaired after wire-catheter advancement, contralateral injection is advisable from the start of the procedure. The contralateral approach can also be achieved by puncturing the same groin with a 4 Fr or 5 Fr diagnostic catheter, which may allow the procedure to be better tolerated. Catheters can then be upsized to 6 or 7 Fr. The operators of the EuroCTO Club used contralateral injection in 62% of cases of their personal series (range 33-78%).
GUIDEWIRES
With a true CTO (> 3 months old) it is unlikely that floppy guide wires will cross the occlusion. The most common mistake by inexperienced operators is to advance the support balloon right against the lesion and force the wire through the lesion, a strategy that almost inevitably results in subintimal wire passage unless a central tapered stump and a very straight occlusion segment are present. A floppy wire is often the best initial choice to negotiate the segment proximal to the occlusion and advance an OTW catheter up to the proximal stump and than exchange to a stiffer dedicated wire. The more aggressive dedicated CTO wires may cause damage to tortuous proximal segments before reaching the occlusion. Some operators routinely select to start a polymer-coated floppy wire (Table 2), which can be used gently to probe the occlusion.

If the wire, supported by the OTW catheter, penetrates the occlusion it may more easily take advantage of the microchannels frequently demonstrated with histology through the occlusion segment13,15. Because of the poor feedback and the lack of resistance offered to advancement, even in a subintimal position (wire-feel) with polymer coated wires, careful attention should be paid with check angiography via contralateral injection to any distal intraluminal wire passage to avoid the creation of long subintimal tracks.
In the context of the frequent case of failure of the initial floppy wire to penetrate the proximal or distal occlusion caps (those sites with greatest resistance in the occluded segment), it is essential to have available an appropriate selection of wires with variable tip stiffness and thickness (regular or tapered), any of which can be selected according to the progress of the procedure. In general, the operator should use only wires with which they are familiar, but this implies a constant exchange of experience as well as personal trials of the new wires as they are available. The majority of operators suggest a step-up approach with wires of moderately increased stiffness at the beginning (Crossit-It 100-200, Miracle 3 or ASAHI medium) with a subsequent switch to wires of greater stiffness and penetration ability, these often being tapered (e.g. Conquest/Confianza wires, Asahi, Japan). Others believe that you can minimise the risk of initial dissection as well as shortening and simplifying the procedure, if you select these stiffer wires initially to achieve passage through a hard occlusion cap (Table 3).
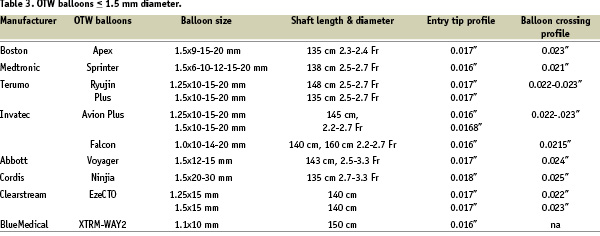
Optimal shaping of the wire tip is essential for its successful crossing. In general, a very small distal curve of approximately 1.0-1.5 mm of 30-45 degrees (primary curve) is needed to penetrate the occlusion entry or distal cap and avoid the creation of large false lumina. For blunt stumps in large vessels, a secondary curve 3-5 mm proximal to the first angle should be fashioned – its size dependant on the vessel diameter – and be used to orientate the tip in the expected direction of the vessel take-off. The wires should be used in combination with a OTW microcatheter or balloon. This allows exchange of a floppy for a dedicated stiffer CTO wire, but also facilitates transmission of torque to the tip and improves feedback. Furthermore, it allows the adjustment of the primary and secondary curves throughout the procedure. A selection of available OTW balloon catheters, ranging from 1.0 mm to 1.5 mm in diameter, is highlighted in Table 2. Dedicated microcatheters (Table 4) may provide a better tip flexibility than OTW balloons and are useful for CTOs immediately distal to a bend (i.e., proximal LCX).
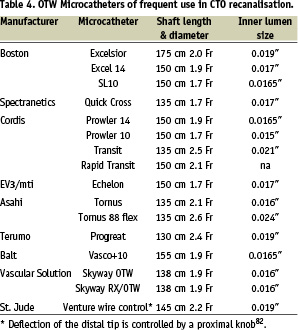
Their larger inner lumen reduces friction during wire manipulation. It is also advantageous to have a radiopaque marker at the very tip of the catheter, to avoid advancing it too far inadvertently, a mistake seen with 1.50 mm balloons which have a single mid-balloon marker. The disadvantage of the microcatheters is that they are rarely able to cross the occlusion and must be exchanged over the wire for small balloons.
THE PARALLEL WIRE TECHNIQUE
Experienced operators carefully check wire progression during penetration of the occlusion, repeating focused angiographic views in multiple projections when the wire appears to have progressed beyond the distal end of the occlusion. If the first wire has entered a false lumen, the parallel wire technique is the best method for locating the true lumen, while minimising the risk of extensive dissection and perforation. The first wire is left in place to mark the dissection channel, and a second wire (typically stiffer and often tapered), with slightly more pronounced primary and secondary curves, supported by an OTW (balloon) catheter, is passed along the same path parallel as the first wire, with care taken to avoid wire twisting. This technique allows for penetration of the distal cap more centrally, guided by the wire left in situ. If the second wire also fails to enter the distal lumen and enters instead a second subendothelial track, often on the opposite wall, the first wire is withdrawn and steered in the direction of the true lumen (the so-called “seesaw” technique)55. Occasionally, three or more wires are used.
It cannot be emphasised enough how essential it is to be absolutely certain that the wire position is correct, especially close to, or just beyond, the distal re-entry. This requires a contralateral injection in all cases without ipsilateral filling. It is not advisable to advance and inject contrast through the OTW catheter to verify the position within the occlusion because, since in case of a false path, contrast may expand the false lumen making it difficult to enter the compressed true lumen. Even if the wire can be advanced easily distal to the occlusion along the expected vessel path, a balloon should not be advanced before the intraluminal wire position is confirmed in 2 orthogonal views by contrast filling the distal bed via collaterals.
STAR (SUB-INTIMAL TRACKING AND RE-ENTRY) TECHNIQUE
This technique was originally developed in peripheral vessels. The expectation was that a sub-intimal wire passage throughout the occlusion segment could offer better results after PTA than long stents, prone to restenosis and thrombosis, or intra-luminal PTA, limited by severe recoil and incomplete expansion56,57. In short, the wire is advanced to a sub-intimal position, and a loop is created and advanced with catheter support through the occluded segment, with multiple re-entries created by balloon dilatation, until finally it is advanced in the true lumen distally. Initial experience has been recently reported in the coronary arteries by Colombo et al79.
There were some safety concerns for higher incidence of perforation compared to other techniques. Another limitation is the potential occlusion of multiple side branches proximal to the distal re-entry, making the technique more suitable for the RCA and marginal branches of the LCX. It also necessitates stenting of longer arterial segments, or if an attempt is made to avoid this, it becomes a two step procedure, both of which translate into higher re-intervention rates. Currently it is not advised as a “first choice” procedure, with the exception of hard impenetrable CTOs, but operators should be familiar with it when a large dissection inadvertently occurs while attempting another technique.
INTRAVASCULAR GUIDANCE OF WIRE ENTRY AND PROGRESSION
Intravascular ultrasound (IVUS) is a valuable tool to detect the correct lumen entry in the absence of an angiographically visible stump58-61. The IVUS catheter can be placed in a side branch and withdrawn to the level of the origin of the occluded vessel, monitoring wire position fluoroscopically and with IVUS. Occasionally advancement of the IVUS catheter in a large dissection plane will facilitate detection of the intraluminal position of a newly progressed wire.
RETROGRADE APPROACH
Recently management of CTOs has been refined through the development of a retrograde approach via collateral pathways and offers an additional possibility of success after failure of antegrade crossing, especially for RCA and LAD occlusions62. In general, this technique is not considered a first line approach and is generally reserved for previous failed attempts. The technique requires specific wire and balloon equipment, and should only be undertaken after training or instruction with an experienced operator. This complex method is applicable when it is deemed possible to pass a wire to the CTO from the collateral donor artery retrograde towards the distal aspect of the vessel (e.g. a septal branch or an epicardial vessel). An easier approach can be offered by the presence of a graft that fills the distal vessel. The steps include the passage of a soft polymer-coated wire (e.g. Whisper or Fielder) via the collateral into the distal vessel which is then steered proximally to approach the distal cup of the occlusion retrograde. The wire can be used as a marker when piercing the distal cap from the antegrade direction, but the real advantage of this technique is the direct penetration of the distal fibrous cap, often less resistant than the proximal cap, and more easily penetrated when coming from a non diseased distal segment than when the wire has already been required to negotiate the occlusion as it does in the antegrade approach. After dilation of the collateral channel with a small balloon (1.00 or 1.25 mm) at low pressure (2-3 atmospheres), a larger OTW balloon is advanced to allow exchange of the soft wire to a stiffer wire which, supported by the balloon inflated in the distal vessel, may allow for retrograde progress through the occlusion. When the distal wire enters a false lumen, a CART-technique (Controlled Antegrade and Retrograde subintimal Tracking) can be used62. The subintimal channel is enlarged by advancing and inflating the retrograde balloon and then a wire is advanced antegrade to penetrate this dissection and link up with the retrograde wire positioned in the distal true lumen.
The review of cases with an unsuccessful antegrade approach suggests that suitable retrograde collaterals are present in more than 50% of cases, but this percentage may be an underestimate if an assessment with a supra-selective injection with a microcatheter of the most promising collaterals is performed, or if dedicated equipment offers safe instrumentation of very tortuous epicardial collaterals.
Dilating the occlusion
Once the stiff wire used to cross the occlusion has been safely advanced into the distal true lumen the operator should consider exchanging this stiff initial wire for a new wire with a softer tip, either by advancing the OTW catheter distally or, less frequently, by advancing the second wire parallel to the initial wire after pre-dilation of the CTO. This is important since sometimes the stiffer wires fail to track in the distal artery and uncertainty can occur as to whether the failure to progress the wire is due to its failure to track or it having lifted a small distal intimal flap.
In a true CTO we expect a fibrotic, often calcified tight lesion, and only very small balloons will be advanced. Recently balloons as small as 1.0, 1.1 and 1.25 mm have been introduced. Lubricious coating, distal tip profile and shaft pushability are as crucial as crossing profile, explaining why sometimes larger balloon catheters may cross when smaller balloons of other brands have failed. If the balloon does not follow the wire, additional techniques to increase the guiding catheter support, such as the use of an anchoring wire (preferably a supportive wire with a soft tip such as the ACS Ironman) or small anchoring balloon63 in a side branch, can be very useful. Advancing through the occlusion another stiffer wire, not necessarily in the distal true lumen, might also facilitate balloon crossing. If the guiding catheter does not provide adequate support, it may have to be exchanged using extension wires, although this may be difficult and may not be the best option when significant time has been spent crossing a CTO with a wire. Occasionally, 2 guiding catheters one inside the other (5 in 6 Fr or 5 in 7 Fr) may combine good passive support from the large external guide and the active support of the smaller inner guide, deeply intubated inside the vessel during balloon expansion. If balloon passage is still not possible, an alternative option is to use rotational atherectomy (Boston Scientific, Natwick, MS, USA). The 0.010” uncoated Rotablator wire can often be passed through the tiny channel created by the guidewire, although this should preferably be done with the OTW balloon as far into the occlusion as possible. If available, an 0.9 mm high density excimer laser catheter (Spectranetics, Colorado, USA) can represent an easier approach because it does not require wire exchange.
The problem of balloon passage will be further reduced when a new device, the Tornus catheter, receives its CE mark. This device, which has already gained wide acceptance in Japan and the US, consists of a braided 2.1 or 2.6 support catheter which can, by using a screwing motion, be advanced along the guidewire and through the occlusion, creating a channel for subsequent balloon passage64.
Once the initial balloon is passed, the subsequent dilatation requires larger balloons. The operator should be advised not to rush this phase of the procedure, as the true size of the distal vascular bed will be obvious only after some time following restored antegrade perfusion pressure. This is important since under sizing the stent might cause later complications such as restenosis and thrombosis. On the other hand, initial over sizing of balloons may cause dissections and unnecessary extension of the treatment site or even perforation.
Stent implantation
In general, all successfully reopened CTOs should be treated with one or more drug-eluting stents. A number of randomised and non-randomised trials have demonstrated a significant reduction in restenosis and re-occlusion as compared to bare metal stents65-72. A mixture of DES and BMS is not advisable, as the restenosis and re-occlusion rate will increase within the BMS treated segment73. The problem remains that in these often diffusely diseased arteries the landing zone for stent placement, which should ideally reach a distal healthy segment, is not easily identifiable. It is often necessary to use long stents, beyond the length of the occluded segment. This practice is now accepted in the DES era, but a “full metal jacket” is still not advisable in small vessels and when there is slow distal flow.
IVUS is also ideally suited to select the appropriate stent size and length in diffusely diseased vessels by measuring the level of remodelling (positive or negative) and the length of the diseased segment. Its usefulness in a CTO setting remains to be proven.
The choice of DES is determined by the proven efficacy of the stent-drug combination, and the stent design allowing the advancement through diffusely diseased and calcific arteries. With today’s stents, failure to advance a DES after appropriate balloon dilatation is rare. Regarding efficacy, DES with very low late lumen loss are probably preferable, as these lesions have a large plaque load and the compression of these plaques within the adventitial space, promotes intimal proliferation and therefore high restenosis and re-occlusion rates as previously demonstrated with BMS74,75.
The ideal duration of combined antiplatelet therapy is unresolved both with any PCI - non-occlusive or occlusive lesions. In the published studies, dual anti-platelet therapy was given for 6 months. Although there is potential for progressive collateral closure and ischaemia after acute occlusion, late events are only rarely reported. The 4% re-occlusion rate with the Cypher stent in the PRISON II study after 6 months may include some late stent thromboses70. In a series of consecutive CTOs treated with Taxus stents, a 3% late stent thrombosis rate was observed beyond 6 months within a 3 year observation period. These events are cause for concern, but due to the great initial benefit, an overall advantage of the DES prevails in CTOs.
CTO devices in current use
The ideal device to facilitate crossing of a CTO would have the capability to combine three major and essential elements: precise steerability, a mechanism to detect and ensure correct intraluminal passage, and the ability to move forward even through fibrotic and calcified lesions, either by mechanical means or using an energy source.
Three dedicated devices are currently in use for CTOs, the Safe Cross-RF™ guidewire (Kensey Nash), the Frontrunner™ Catheter (Lumend, Redwood City, CA, USA) and the Crosser system (FlowCardia, CA, USA).
The Safe Cross-RF™ Guidewire is a 0.014” intermediate-stiffness guidewire, emitting and receiving near infrared light (optical coherence reflectometry, OCR). In OCR, spectroscopic analysis of the near infrared light reflected from the plaque or vessel wall allows a distinction of these two structures based on their homogeneity. An elementary green and red display warns the operator when the wire tip is advanced within 1 mm of the outer vessel wall and automatically stops firing the radiofrequency energy.
This device was tested in the multicentre Guided Radio Frequency Energy Ablation of Total Occlusions (GREAT) Registry, a prospective non-randomised multicentre study in 116 patients with CTOs refractory to a 10 minute attempt with conventional guidewires76. Device success (wire advancement in the distal lumen) was achieved in 54.3% (63 of 116 lesions), and was independent of vessel location, occlusion duration, lesion morphology and collateral type. There were no procedure-related deaths, Q-wave myocardial infarctions, or emergency bypass operations. Clinical perforation occurred in 3 patients (2.6% overall), 1 (0.7%) of which one was adjudicated to be directly related to the SafeCross-RF™ wire.
The Frontrunner Catheter is designed to create and propagate an intra-luminal pathway through a CTO via blunt micro-dissection77. A bilaterally hinged distal tip assembly is manually opened and closed as the device is worked across the occlusion. A probing and recanalisation catheter (the 4.5 Fr Micro Guide Catheter) provides support for the device as it operates. It was applied in 593 patients using the current X-39 Frontrunner (0.03” to 0.04” outer diameter, with a 2.8 Fr distal tip). Lesion success (ultimate intra-luminal placement of a conventional guide wire) was achieved in 61% of cases. Perforations were observed in 0.9% of cases.
The CROSSER™ CTO recanalisation system (Flow-Cardia Inc, CA, USA) is comprised of a generator, transducer, foot switch, and a disposable catheter. The generator applies AC current to piezoelectric crystals, resulting in their expansion and contraction within the transducer. The transducer then converts, amplifies, and transmits this energy to the catheter, which results in vibration of the tip at a rate of 21,000 cycles/sec. This vibration provides mechanical impact and cavitational effects, which supposedly aid in the recanalisation of the occluded artery. The catheter is monorail, hydrophilic, and can be advanced over a standard 0.014 inch guidewire. It is 1.1 mm in diameter, which makes it compatible with 6 Fr guiding catheters and has a blunt tip. A minimum vessel diameter of > 2.5 mm is recommended. The infusion lumen of the CROSSER™ allows it to inject diluted contrast to confirm its intraluminal position. An irrigation line is required for continuous sterile saline flush through the CROSSER™ during device activation, which provides for cooling of the system and offers a medium to facilitate cavitation at the catheter tip. In a small single centre experience, 28 patients (30 lesions) were included. The median age of the CTO was 9 months (range 3-60 months). Technical success was obtained in 63% of the occlusions with minor complications78.
When to stop the recanalisation procedure
Several variables will determine when to bring to an end attempts to cross the occlusion. Excessive dye use (typically around 600 mls in a non-diabetic patient with normal renal function; much less in patients at risk for contrast nephropathy) should strongly suggest to the operator that is is time to end the procedure. Procedural events such as the creation of a large false lumen may render further wire manipulations futile: a second attempt at another time may have better chances if performed 4 or more weeks after to allow vessel wall healing. The wire manipulation may also cause intramural haematomas resulting in loss of visualisation of the distal vessel via collaterals. Here, as well, a second subsequent attempt can be considered. Last, but not least, excessive patient or operator fatigue may necessitate stopping the procedure, and possibly planning a second attempt. Persistent subintimal contrast staining is not necessarily a reason to stop: a parallel wire or STAR technique may still be successful79. A second attempt after a failed CTO is successful in > 50% of patients, especially when the mode of failure is understood and a feasible alternative strategic approach has been formulated, including a possible retrograde approach.
Consensus on technique
1. Angiographic and patient related characteristics allow a distinction between “simple” chronic occlusions, with a probability of success higher than 90% and “complex” CTOs, with a success rate as low as 50-60%.
2. CTO remains one of the few conditions in which 7 and 8 Fr catheters may still have a role in complex cases when a parallel wire technique or identification of the entry point with IVUS are required.
3. Over-the-Wire catheters should be used routinely in CTO recanalisation because they allow switching from floppy wires, to negotiate the vessel up to the occlusion, to stiff dedicated wires with an appropriate distal curve and to go back to “normal” floppy wires after crossing.
4. The retrograde approach to CTO is very promising, but it requires specific training and equipment and at this stage should not be attempted outside a selected number of pilot centres.
5. None of the new CTO devices has shown better success rates than conventional wires and balloons.
6. DES should be implanted in all cases after successful recanalisation of a CTO.
Conclusions
There is overwhelming evidence that very low restenosis and re-occlusion rates can be obtained with DES after recanalisation of CTO65-72, and there is emerging evidence that persistent patency after percutaneous recanalisation of chronic coronary occlusions results in improved survival, enhanced left ventricular function, reduced angina and improved exercise tolerance28,29. PCI should be considered the preferred initial revascularisation modality in patients in whom a high procedural success rate may be anticipated. Fortunately, in the last years advances in guidewire technology and the development of innovative techniques have resulted in success rates of > 70-80%, allowing for experienced operators to also consider PCI for very complex CTOs.
There is certainly need for further technical developments. A forward looking imaging modality would be extremely helpful in guidewire advancement. The integration of tomographic high-resolution imaging techniques such as multislice CT imaging into the intra-procedural fluoroscopic image may also offer a helpful indication of the presumed vessel path80,81.
The slow acceptance in Europe of the need for standardised dedicated training in interventional cardiology, along with the concept that every cardiologist should be allowed to perform any angioplasty procedure with no specific previous experience as well as an insufficient ongoing workload, are the main obstacles to the development of an appropriate practice based on the concentration of cases and super-specialisation, these very aspects that have made treatment of CTO so successful in Japan. In this paper we have tried to indicate the basis for changes in training, work organisation and practice to re-establish clinical excellence in the treatment of this challenging condition.
Acknowledgement
The authors wish to thank their Japanese colleagues who promoted the advancement of CTO treatment in Europe by kindly sharing their experience and demonstrating their technique in live courses; specifically (in alphabetic order) Osamu Katoh, Kazuaki Mitsudo, Masahiko Ochiai, Shigeru Saito, Takahiko Suzuki, Hideo Tamai. Also they wish to acknowledge Mr. Ad den Boer for expert counsel on radiation safety.

