Abstract
Background: Chronic mesenteric ischaemia (CMI) is associated with a high morbidity and mortality. Most likely causes of CMI are atherosclerosis, external compression (Dunbar Syndrome) and vasculitis. We report on our experience with endovascular stent therapy. Patients and Methods: Between Sept. 1998 and Dec. 2005 we treated 21 consecutive patients (52% male; mean age 64±15 years, range 18-81 years) due to symptomatic CMI. A total of 34 interventions with 41 treated lesions were performed. Aetiology of vessel obstruction was atherosclerosis in 14 patients (67%), Dunbar syndrome in 3 patients (14%), chronic type B aortic dissection in 2 patients (9%), Takayasu’s arteritis in 1 patient (4.5%), external compression following abdominal surgery in 1 patient (4.5%). Target lesions were celiac trunk (n=16), superior mesenteric artery (n=24), and inferior mesenteric artery (n=1). Twenty-two percent (n=9) of the lesions were total occlusions. The patients underwent serial follow-up duplex examinations before discharge and every 6 months thereafter or in case of symptom recurrence.
Results: Thirty-one of the 34 (91%) interventions were technically successful (37/41 lesions; 90%). Two of these 4 lesions were treated successfully using another approach in a second intervention. Brachial access was used in 16/34 cases (47%). One or more stents were placed in 29/34 interventions (85%) and 35/41 lesions. In 3 cases, in-stent restenosis was treated with balloon angioplasty alone, and in two cases, 3 occlusions could not be reopened. During 6 interventions, drug eluting stents were placed. All patients treated successfully were free of symptoms immediately after the intervention with recurrence of symptoms in case of restenosis. After a mean follow-up of 31±26 (range 0-87) months restenosis was detected in 6 patients (29%). Two major complications occurred which were treated without permanent sequelae.
Conclusion: Stenoses of mesenteric arteries resulting in symptomatic CMI can be treated successfully with stent-angioplasty; for anatomical reasons, the brachial approach should be considered. Recanalisation of total obstructions is feasible if a stump of the occluded artery is detectable. Restenosis is frequent and can easily be treated with balloon angioplasty or stent-in-stent placement.
Introduction
Acute mesenteric ischaemia is mostly caused by an embolism which produces a bowel infarction and has a typical acute onset of diffuse abdominal pain with a reported mortality rate of 70-90% despite immediate surgery or endovascular therapy1-4. In contrast, chronic mesenteric ischaemia (CMI), independent of its aetiology, is characterised by frequently unrecognised and unspecific symptoms such as: diffuse postprandial abdominal pain, diarrhoea induced by ischaemic enteritis, and unintended weight loss5,6. Sometimes years will pass before the right diagnosis is made7. Surgery performing thrombendatherectomy or bypass is the established therapy2. Endovascular therapy using balloon angioplasty (PTA) of atherosclerotic mesenteric artery stenoses has the same limitations as PTA of atherosclerotic renal artery stenoses with a limited technical success rate and high restenosis rate8; stenting of ostial lesions improves the acute success rate, avoiding acute recoil and flow limiting dissection9-18. We report on the acute and long-term outcome of a cohort of patients with different aetiologies of CMI undergoing stent-angioplasty.
Patients and methods
Between Sept. 1998 and Dec. 2005 we treated 21 consecutive patients (52% male; mean age 64±15 years, range 18-81 years) due to symptomatic CMI (patient demographics are plotted in Table 1a & 1b). All patients suffered from postprandial abdominal pain, and 7 of 21 (33%) of unintended weight loss. A total of 34 interventions with 41 treated lesions were performed. Aetiology of vessel obstruction was: atherosclerosis in 14 patients (67%), Dunbar syndrome (Figure 1) in 3 patients (14%), chronic type B aortic dissection in 2 patients (9%), chronic non-active Takayasu’s arteritis (Figure 2) in 1 patient (4.5%), extrinsic compression following abdominal surgery in 1 patient (4.5%). In addition, 12 interventions were done in cases of restenosis after primarily successful (stent)-angioplasty. Target lesions were: celiac trunk (n=16), superior mesenteric artery (n=24), and inferior mesenteric artery (n=1). Twenty-two percent (n=9) of the lesions were total occlusions.
The patients underwent serial follow-up duplex examinations before discharge and every 6 months thereafter or in case of symptom recurrence.
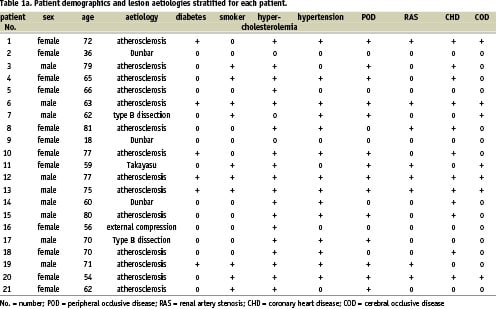
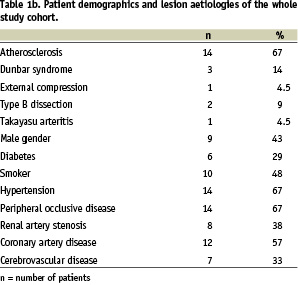
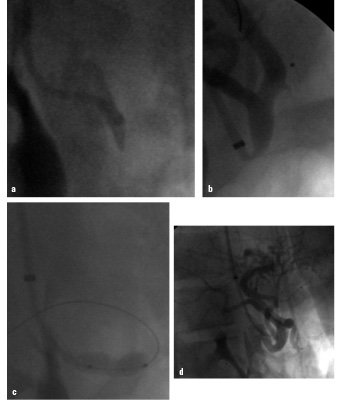
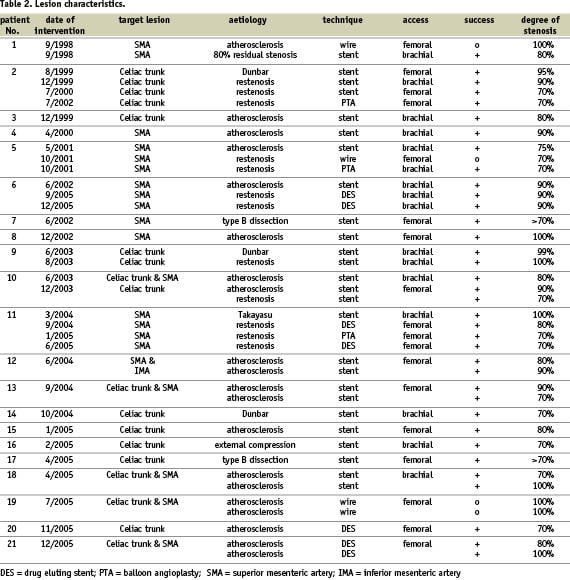
Figure 1. 18-year old female patient with Dunbar syndrome: a) Proximal occlusion of the celiac trunk by extrinsic compression; b) “recanalisation” with a 0.014” Pilot 150 wire via brachial approach; c) 5/16 mm balloon angioplasty, persisting balloon impression by the arcuate ligament; d) final result after 5/16 mm Multilink Ultra™ stent.
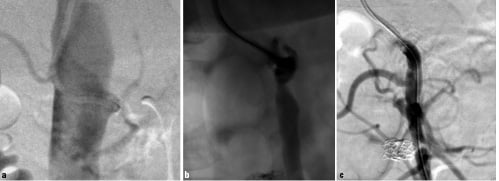
Figure 2. 59-years old female patient with chronic inactive state of Takayasu’s disease: a) chronic total occlusion of the SMA; b) after subintimal recanalisation with a 0.9 mm excimer laser; c) final result after placement of a 4/33 mm Multilink Zeta™ stent.
Technique of intervention
All interventions, regardless of whether or not they used the brachial or femoral approach, were performed using the guiding catheter technique. After placement of a 6 French 11 cm long sheath (Avanti™, Cordis, Haan, Germany), the target vessel was cannulated using a 6F guiding catheter: via the femoral approach internal mammaria artery (IMA), renal double curved (RDC), or Hockey Stick configurations were used, and for the brachial access exclusively a multi-purpose configuration. In stenotic lesions, a 0.014’’ guidewire (Galeo ES, Biotronik, Berlin, Germany or Pilot™ 150, Guidant Europe, Diegem, Belgium) was placed across the lesion followed by a predilatation of the lesion with a 3/20 mm monorail balloon catheter (Maverick, Boston Scientific, Ratingen, Germany). After predilatation, various types of coronary or renal stents were implanted all being rapid exchange devices. In occlusions, crossing of the CTO was attempted using various types of 0.014’’ coronary guidewires or using a 0.035 guidewire (Terumo, Leuven, Belgium) which was later replaced by a 0.014’’ extra support wire. Predilatation was performed using different balloon sizes varying from 1.5 mm to 3 mm. In one patient, because of inability to penetrate a chronically occluded stump of the SMA, a 0.9 mm excimer laser catheter (Spectranetics, Denver, Colorado, USA) was used to create a short channel into the occlusion which could then be crossed with a guidewire.
Medical therapy
Post-interventional therapy consisted of long-term therapy with aspirin 100 mg/day and clopidogrel, 75 mg/day, for 4 weeks; in the case of implantation of a drug eluting stent this is extended to 6 months. The antiplatelet therapy was started at the very latest, the day before the intervention with a loading dose of clopidogrel of 600 mg. Peri-interventional administration of 2500-5000 IU of heparin is given intra-arterially after sheath placement.
Statistics
Continuous variable values are given as the average value ± standard deviation or as the number of patients or lesions and percent. Technical success was defined as a residual stenosis <50% and procedural success as a residual stenosis < 30%. Kaplan-Meier analysis was applied to calculate cumulative restenosis free survival.
Results
Table 2 summarises the baseline and outcome lesion characteristics. Thirty-one of the 34 (91%) interventions were technically successful (37/41 lesions; 90%). All lesions were located ostial, defined as within 10 mm distance to the origin in atherosclerosis, vasculitis and dissection cases or in the proximal segment of the celiac trunk in case of external compression syndromes. Brachial access was successfully used in 16/34 cases (47%), the femoral one in the remaining 18 cases (53%), 3 of these interventions with 4 lesions were unsuccessful. One or more balloon expandable stents were placed in 28/34 interventions (82%) and 34/41 lesions. During 6 interventions, drug eluting stents were placed (one 3.5 mm Cypher™ stent, Cordis Corp., Miami, Florida, USA, which was post-dilated with a 5 mm Quantum™ balloon catheter, Boston Scientific, Ratingen, Germany; in the remaining 5 cases, 5 mm Taxus Express™ stents, Boston Scientific, Ratingen, Germany, were implanted). In 3 cases, in-stent restenosis was treated with balloon angioplasty alone: in 1 patient, 2 occlusions could not be reopened because of an angiographically undetectable stump of the occluded SMA and celiac trunk; two other primarily unsuccessful treated SMA lesions could be stented successfully using a brachial approach in a second intervention after the initial failed femoral approach.
Of the 9 total occlusions, 6 (67%) could be successfully reopened and stented with another occlusion successfully stented in a second approach ending up in a total success rate of 78% for total occlusions. In one of these total occlusions (chronic non-active Takayasu`s arteritis), a 0.9 mm excimer laser probe (Spectranetics, Denver, Colorado, USA) had to be used to penetrate the chronically occluded stump of the SMA and to engage the vessel lumen with the guide wire (Figure 2).
All patients treated successfully were free of symptoms immediately after the intervention, with recurrence of symptoms in case of restenosis. After a mean follow-up of 31±26 (range 0-87) months, restenosis was detected in 6 patients (29%). All restenoses were detected with duplex ultrasound demonstrating at least a three fold increase in peak systolic flow velocity (range 3.0 to 5.6) compared to the immediate post-interventional examination. All patients with restenosis complained about the recurrence of postprandial abdominal pain. The duplex diagnosis was in all cases confirmed be angiography showing at least 70% restenosis. Restenosis was detected in 3/14 patients (21%) with atherosclerotic lesions, in 2/3 (67%) of the patients with Dunbar syndrome, in the one patient with Takayasu’s arteritis and in none of the 2 patients treated with type B aortic dissection. Two restenoses were detected in lesions treated with drug eluting stents, both at the proximal or distal margin of the stents. Angiographically, restenosis was located in both cases in non stent-covered segments of the lesions and was retreated with the implantation of a second drug eluting stent (Figure 3).
Figure 3. 63-year old male patient with atherosclerotic recurrent stenosis of the SMA (celiac trunk and IMA chronically occluded): a) Focal tight ostial stenosis proximal to the recently implanted 5/32 mm Taxus Express™ stent; b) overlapping placement of a 5/12 mm Taxus Express™ stent; c) final result.
Recurrent stenosis was found in 2 of the 4 patients with extrinsic compression of the celiac trunk, three times caused by the arcuate ligament (Dunbar syndrome) and in one patient following multiple abdominal surgeries as a consequence of a severe car accident. These 2 patients had subtotal occlusion at baseline; recurrent stenoses despite repeated endovascular procedures led to surgical decompression in both patients. Figures 4 a & b show the restenosis-free Kaplan-Meier survival curves for the whole study cohort and stratified to atherosclerosis, Dunbar syndrome and others show no significant differences in occurrence of restenosis (log rank 0.304).
Two patients died during follow-up because of coronary artery disease.
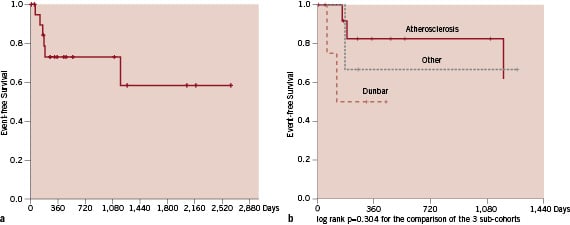
Figure 4 a and b. Kaplan-Meier survival curves for restenosis free survival for the whole study cohort (a) and stratified to the aetiologies of stenotic-occlusive mesenteric disease (b).
Complications
Two major complications occurred; a retroperitoneal bleeding induced by a perforation of an intrarenal segment artery induced by wire misplacement needing coil embolisation without any sequelae, and a thrombotic occlusion of the access site artery (brachial artery) which was reopened by endovascular means the day after target lesion intervention.
Discussion
We are reporting here our single centre experience with consecutive endovascular treatment of patients with symptomatic chronic mesenteric ischaemia (CMI). Endovascular intervention offers promising acute and long-term success rates with a low complication rate and even in case of restenosis endovascular re-treatment is feasible.
Chronic mesenteric ischaemia is characterised by painful abdominal cramps and colic, typically postprandial (abdominal angina), which is accompanied by weight loss5-7. In some cases there may also be atypical symptoms like nausea, vomiting, diarrhoea, and bloody stool frequently responsible for a delay of the diagnosis7. These symptoms are the clinical expression of the motor excitation of the intestines as a result of chronic ischaemia.
The viscera are supplied by the celiac trunk, the superior mesenteric artery, and the inferior mesenteric artery. Adequate blood supply to the intestines is controlled by the physiological cooperation of the hormonal, nervous, and vascular systems, forming a complex auto regulatory mechanism19. The clinical symptoms of abdominal angina appear usually when two of the three visceral arteries are finally obstructed. However, there are reports of asymptomatic patients with occlusion of all three visceral arteries, as well as patients with abdominal angina who have only one vessel involved20. The clinical status of the patients is determined by collateral circulation and the auto regulation system21. The most common aetiological factor causing chronic mesenteric ischaemia is atherosclerosis; an autopsy study5 showed that the occurrence of mesenteric artery stenosis was strongly associated with ageing. Sixty-seven per cent of the subjects aged 80 or more presented with mesenteric artery stenosis, whereas the rate was 6% among those aged less than 40 years. Despite sometimes extensive stenotic alterations in the mesenteric arteries, only one patient in this study had bowel necrosis at autopsy. As a part of general arteriosclerotic process of the circulatory system, mesenteric artery atherosclerosis is strongly associated with atherosclerosis in coronary arteries and with atherosclerosis of cerebral arteries in the skull base. This correlation of atherosclerosis of the splanchnic vessels with coronary artery disease was also found in our study (79%) and explains – besides the age of this patient cohort – the high reported mortality rate of 40%, even in patients with initially asymptomatic CMI6. Other, more infrequent causes of CMI are fibromuscular dysplasia, Takayasu’s disease, or aortic dissection type B22-25. A small number of cases of abdominal angina, primarily in young women, may result from compression of the celiac trunk by the arcuate ligament (Dunbar syndrome)26.
Surgery was, for many years, the standard method of treating stenosis of the visceral arteries.
Perioperative mortality ranged from 3% to 20%, while in 15% of cases occlusion of the surgically treated arteries was found after 38 months of follow-up27,28. Comparable to the treatment of atherosclerotic renal artery stenosis, the introduction of low profile stent devices has made endovascular therapy the method of choice in the treatment of stenosis of the visceral arteries8-10,13,14,29. It is difficult to compare the clinical and technical long-term outcome for the treatment of stenosis of the visceral arteries using stents due to the small number of reports. The present study found excellent acute results, both technically and clinically, with a complete relief of symptoms in all studied patients. Long-term patency, especially of atherosclerotic lesions, is acceptable considering the fact that using drug eluting stents has the potential to further reduce the 21% restenosis rate.
The present study included four patients with extrinsic compression of the celiac trunk, three times caused by the arcuate ligament (Dunbar syndrome) and in one patient following multiple abdominal surgeries as a consequence of a severe car accident. In the two patients with subtotal occlusion at baseline, recurrent stenosis – despite repeated endovascular procedures including stent-in-stent application – led to surgical decompression. The cause of restenosis in these 2 patients was stent compression. As a consequence, stenting of extrinsic compressed visceral arteries should only be performed in surgical high risk patients.
In the two lesions treated with drug eluting stents that developed restenosis in our series, restenosis was located at the margins of the stents suggesting that the stents did not cover the whole lesion initially. All restenosis were detected with duplex ultrasound demonstrating at least a three fold increase in peak systolic flow velocity compared to the immediate post-interventional examination. However, all patients diagnosed with restenosis presented with a re-occurrence of postprandial abdominal pain making the diagnosis of restenosis more likely.
In about half the interventions, we preferred the antegrade trans-brachial approach due to anatomical reasons. Especially considering the take-off of the superior mesenteric artery, which is mostly characterised by a steep angle making it sometimes difficult, or even impossible, to place a guidewire or place a stent around the curve using the femoral approach. In two cases of a failed femoral approach to treat a superior mesenteric artery lesion, it was found to be relatively easy to succeed coming from the brachial approach. The take-off of the celiac trunk is usually easier to treat from the femoral approach, however, especially in patients presenting with Dunbar syndrome, the course of the artery is s-shaped and usually easier to treat from the brachial access.
In conclusion, considering the reported high mortality rate of patients even with asymptomatic CMI and significant three-vessel mesenteric arterial disease, prophylactic surgical or endovascular mesenteric arterial reconstruction should be considered. Mesenteric arterial reconstruction should be routine when these patients undergo aortic reconstruction for aneurysmal or occlusive disease. In the remaining patients, endovascular interventions offer promising acute and long-term success rates with low complication rates. Because of the high coincidence of significant coronary artery disease in these patients, coronary angiography should be considered. The characteristic triad of postprandial abdominal pain, food aversion, and weight loss in an elderly patient along with other manifestations of arteriosclerosis should suggest a possibility of the visceral arterial insufficiency.
A major limitation of this study is its observational character, a retrospective study with a relatively small number of patients including different aetiologies of CMI. Furthermore, treatment strategies were not randomised. A randomised multicentre study comparing balloon angioplasty with the placement of bare metal or drug eluting stents within a clearly defined patient cohort presenting with atherosclerotic mesenteric stenoses would be valuable.

