Abstract
Aims: We evaluated the effect of cyclooxygenase-2 inhibitor rofecoxib added to atorvastatin, initiated immediately after percutaneous coronary angioplasty (PCI), on C-reactive protein (CRP) and interleukin-6 (IL-6) in patients with unstable angina (UA).
Methods and results: Sixty patients were prospectively randomized to receive: (A) 10 mg atorvastatin or (B) 40mg atorvastatin or (C) atorvastatin 40 mg plus rofecoxib 12.5 mg. Treatment was continued for 6 months. CRP and IL-6 levels increased within 48 hours after PCI in all groups, in comparison with A and B, a more pronounced decrease in CRP (mg/l) and IL-6 (pg/ml) levels at 3- and 6-month follow-up was present in C: CRP at 6 months: 0.76 vs. 1.92 vs. 0.11, p<0.05; IL-6 at 6 months: 0.02 vs. 0.04 vs. 0.01, p<0.05. At 6-month follow-up the reduction in CRP level occurred in 70% of subjects with CRP higher than median (3.6mg/l) at baseline vs. 15% of patients with CRP lower than median (p<0.041).
Conclusion: After PCI in UA, cyclooxygenase-2 inhibitor rofecoxib added to atorvastatin reduced CRP and IL-6 levels more profoundly than atorvastatin alone at 3- and 6-month after intervention. In the group receiving combined therapy, reduction in CRP levels was more frequently observed in patients with higher CRP at baseline.
Background
Atherosclerosis is an inflammatory disease1. The elevated levels of inflammatory markers such as C-reactive protein and interleukin-6 are useful predictors of the cardiovascular adverse events in a variety of patient populations2. Elevated C-reactive protein and interleukin-6 in patients presenting with unstable angina also predict adverse long-term clinical outcomes3,4. Moreover, in patients with unstable angina and initially elevated C-reactive protein on admission, percutaneous coronary angioplasty causes a further rise in the inflammatory markers5. The magnitude of preprocedural C-reactive protein elevation also correlates with increased incidence of short- and long-term adverse cardiac events, as well as with restenosis after percutaneous coronary angioplasty6.
However, little is known whether the elevated levels of serum inflammatory markers induced by angioplasty can be effectively lowered by drugs having potential anti-inflammatory properties, resulting in reduction of late adverse cardiovascular events. Of such drugs, statins have long been deemed to exert their salutary effect on cardiovascular adverse events by attenuating the inflammatory processes and thus stabilizing the atherosclerotic plaque7. Most recently, statins have been shown to help the rapid transformation of unstable coronary artery disease into a stable condition with a very low event rate over the forthcoming 6 months in patients with unstable angina undergoing successful coronary stent implantation8. Consequently, we tried to determine if addition of an anti-inflammatory drug, a selective cyclooxygenase-2 inhibitor rofecoxib, could modify the effect of statin on elevated inflammatory markers when immediately initiated after percutaneous coronary angioplasty for unstable angina.
Material and methods
Study population
Sixty patients were enrolled in a randomized, prospective, unblinded, three armed, parallel design study at the Institute of Cardiology, Jagiellonian University Medical College, Krakow, Poland. The protocol was approved by the Institutional Review Board, the patients gave informed consent. The patients with unstable angina (class IIB or IIIB), significant coronary stenosis qualified for percutaneous coronary angioplasty, and hypercholesterolemia without previous statin therapy were randomized to: group A receiving atorvastatin 10mg/day, group B receiving atorvastatin 40mg/day or group C receiving atorvastatin 40 mg/day concomitantly with rofecoxib 12,5 mg/day. Treatment was started immediately following percutaneous coronary angioplasty and continued for 6 months. Patients with known chronic inflammatory disease were excluded. We treat hypercholesterolemia according to the current guidelines9. We decided not to include a placebo group or to enroll patients without known hypercholesterolemia.
Blood sampling
Blood samples were obtained at: baseline (within no more than 7 days from index event), 48 hours, 3 months and 6 months after percutaneous coronary angioplasty. Levels of total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides were assayed. Levels of C-reactive protein were measured by a high sensitivity assay (Dade Behring, Germany). Interleukin-6 levels were measured using immunoassay (R&D Systems, USA).
Statistical analysis
Statistical analyses were performed by a biostatistician using Stata 5.0 software. Because variables such as C-reactive protein, interleukin-6, triglycerides were skewed, comparisons between groups were carried out using nonparametric tests (the Mann-Whitney or Kruskal -Wallis tests or Wilcoxon test for paired variables as appropriate). Variables with normal distribution (total cholesterol, LDL cholesterol, HDL cholesterol) were compared by means of the paired t test. Patients were divided into two groups with low and high C-reactive protein levels according to median value and this division by median value was data-driven. We computed absolute change in C-reactive protein levels for baseline and 6-month follow-up, a process that resulted in a normal distribution of values, so the Pearson correlation coefficient was used to test the relations between variables. The level of statistical significance was set at P<0.05.
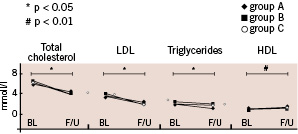
Figure 1. Effects of the three therapy regimens on lipid profile. Total cholesterol, LDL and triglycerides decreased, and HDL increased significantly from baseline (BL) to 6-month follow-up (F/U), but the magnitude of effect was similar in all three groups. Group A: 10 mg atorvastatin, B: 40 mg atorvastatin, C: atorvastatin 40 mg plus rofecoxib 12.5 mg.
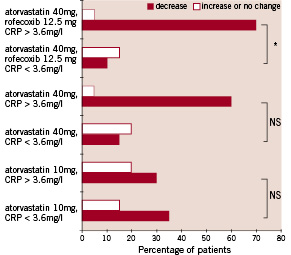
Figure 2. Effect of the three therapy regimens on changes of C-reactive protein level from baseline to 6-month follow-up as related to the initial C-reactive protein level: higher than median (>3,6 mg/l), and lower than median (<3,6 mg/l). The percentage of patients who demonstrated suppression of C-reactive protein level is presented in solid bars, the percentage of patients who experienced decrease or no change of C-reactive protein level is shown in open bars. In the group receiving combined atorvastatin and rofecoxib, patients with baseline C-reactive protein level higher than median experienced suppression significantly more frequently than those with low baseline C-reactive protein levels (70% vs. 15%, * p<0,041 for group C). A similar trend could also be observed for atorvastatin 40 mg, but the differences between subgroups of groups A and B were not significant.
Results
Baseline clinical and demographic data are presented in Table 1.
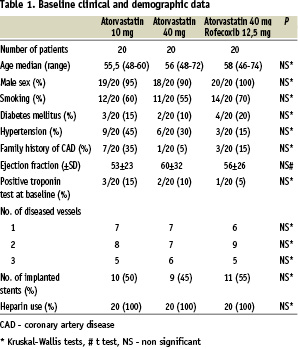
The three groups did not markedly differ at baseline with regard to age, sex, smoking, diabetes mellitus, hypertension, family history of coronary artery disease, ejection fraction, positive troponin test at baseline, number of diseased vessels, number of stents implanted or use of heparin. Patients were taking the following medications at discharge: aspirin n=60 (75 mg), ticlopidine n=60 (250 mg bid), ACE inhibitors n=45, beta-blockers n=41, calcium channel blockers n=32, nitrates n=12. No differences between study groups were observed.
Major adverse cardiovascular events were defined as cardiovascular death, nonfatal myocardial infarction and urgent repeat percutaneous coronary angioplasty. No major adverse cardiovascular events occurred in-hospital or during prespecified 6-month follow-up. The repeat target vessel revascularization rate for the duration of the study was 20% (n=4) in the atorvastatin 10mg group, 15% (n=3) in the atorvastatin 40mg group and 15% (n=3), in atorvastatin 40 mg plus rofecoxib 12.5 mg group. No adverse effects of atorvastatin were recorded (including hypersensitivity, rhabdomyolysis, liver dysfunction, renal failure). We also closely monitored for possible adverse effects of rofecoxib. Treatment with rofecoxib was uneventful and none of the specific adverse effects (thrombosis, aggravation of hypertension, gastrointestinal bleeding or symptoms) occurred. No changes in serum creatinine levels were observed between baseline and 3-month and 6-month follow-up in any of the groups.
Total cholesterol, LDL cholesterol and triglycerides decreased to similar extent in all groups at 6-month follow-up. HDL cholesterol levels increased at 6-month follow-up, also without differences in magnitude between groups (Fig. 1).
Table 2 summarizes C-reactive protein levels at baseline, 48 hours after percutaneous coronary angioplasty and at follow-up.
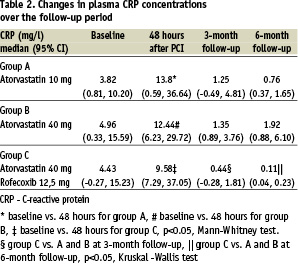
Median C-reactive protein levels were significantly increased 48 hours after the procedure in all groups. All groups showed reduction in median C-reactive protein levels at 3-month and 6-month follow-up in comparison with baseline. However, no dose-dependent effect of atorvastatin on median C-reactive protein levels was observed (group A vs. B, p<0.84 and p<0.63, for 3- and 6-month follow-up, respectively). Concomitant treatment with atorvastatin and rofecoxib significantly reduced median C-reactive protein levels in comparison with group A and B (Table 2).
We used the median value of C-reactive protein (3.6 mg/l) to divide each group according to initial C-reactive protein into subgroups with higher and lower baseline C-reactive protein level. We then calculated the percent of patients who presented with decrease vs. no change/increase in C-reactive protein levels from baseline to the 6-month follow-up in each subgroup. This enabled us to link the long-term drug effect on C-reactive protein suppression to the baseline inflammatory status. At the 6-month follow-up, reduction in C-reactive protein level was more frequent in subjects with higher (>3.6 mg/l) than in those with lower (<3.6 mg/l) C-reactive protein levels at baseline (70% vs. 15% of patients with decreased C-reactive protein, p<0.041). This effect, however, was only observed in group C receiving combined anti-inflammatory treatment (Fig. 2).
Median interleukin-6 levels were increased 48 hours after the procedure (Table 3).
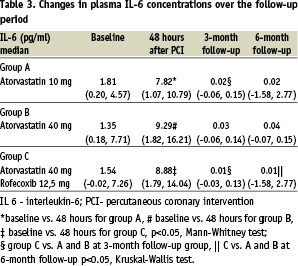
The patients treated with atorvastatin (10 and 40 mg) had reduced interleukin-6 levels at 3 and 6 months, but no dose-dependent effect was observed. Concomitant treatment with rofecoxib significantly reduced median interleukin-6 levels when compared to both doses of atorvastatin at the 3-month and 6-month follow-up.
No significant correlation was found between differences in C-reactive protein level and differences in total (r=0.123, P=0.40), LDL (r=-0.104, P=0.48), HDL (r=0.080, P=0.59) cholesterol or triglycerides (r=-0.121, P=0.41) measured at baseline and 6-month follow-up. Moreover we did not find any significant correlation between IL-6 and lipid parameters and between the change in CRP and the change in IL-6.
Discussion
The main findings of the present study are: 1) six months after percutaneous coronary angioplasty for unstable angina in hyper-cholesterolemic patients, atorvastatin reduced C-reactive protein and interleukin-6 serum levels elevated at baseline, regardless of the statin dose; 2) rofecoxib added to atorvastatin therapy induced even more profound suppression of these inflammatory markers; 3) in the group receiving combined anti-inflammatory therapy, the reduction in C-reactive protein serum levels was most frequent in patients who had most elevated C-reactive protein levels at baseline, suggesting that such patients may benefit most from aggressive anti-inflammatory therapy.
Elevated serum C-reactive protein levels have been consistently found in patients with different forms of atherosclerotic disease. In patients with acute coronary syndromes elevated levels of C-reactive protein on admission indicate increased risk for recurrent cardiovascular events2. Increased C-reactive protein levels in patients undergoing angioplasty or stenting are also strongly predictive of in-hospital and long-term thrombotic events as well as of increased risk for restenosis6,10-12. Furthermore, patients with unstable angina and chronically elevated C-reactive protein are at higher risk for recurrent cardiovascular events than their counterparts with normalized C-reactive protein levels at discharge and during the follow-up13. Administration of anti-inflammatory drugs may prove to be a viable therapeutic goal for patients with unstable angina14.
Several groups of drugs hold promise to blunt the inflammatory hyperreactivity in patients with atherosclerosis. Among these drugs, statins have the most extensive record of both reducing inflammatory markers15-18 and improving survival in patients with clinically manifest atherosclerosis as well as asymptomatic individuals with hypercholesterolemia19. Statins have been shown to exhibit an array of biological effects beyond cholesterol lowering. It is possible that the mortality and morbidity benefit of statins, especially in patients undergoing coronary angioplasty, is causally linked to their anti-inflammatory actions7,20. Statins have been shown to attenuate the expression of the cytokines (interleukin-1, interleukin-6), cellular adhesion molecules as well as to reduce the conversion of monocytes to macrophages and T-cell activation2. Also, the reduction of C-reactive protein levels during statin therapy is a rapidly occurring class effect and seems to be unrelated to the dose of statins16,17. In our study, the change in C-reactive protein was related neither to total, LDL, or HDL cholesterol nor to the triglycerides level, which confirms previous findings by Ridker et al. that less than 2% of the variance in C-reactive protein can be explained by changes in lipid profile17. This characteristic of statins to blunt the enhanced inflammatory response in atherosclerosis suggested that the potential benefit from statins could be largest when drugs would be started early after acute coronary event. Accordingly, the recent expansion of statin use to early administration after acute coronary syndromes including myocardial infarction succeeded in reducing recurrent ischemia21 or even 1-year mortality22. Most recently, statins have been demonstrated to help the rapid (in the first 4 weeks of therapy) transformation of unstable coronary artery disease into a stable condition with a very low event rate over the following 6 months in patients with unstable angina after stent implantation8. In PROVE IT-TIMI 22 Study an intensive statin therapy provides greater protection against cardiovascular events then does a standard statin therapy. Additionally intensive, high dose statin therapy has resulted in more pronaunced CRP level reduction. In this study CRP level < 2 mg/L at day 30 after ischemic event was associated with improved outcomes after two years regardless of LDL values23. Accordingly, the present study confirms that administration of statin to patients with unstable angina, in whom enhanced inflammatory response is further exacerbated by percutaneous coronary angioplasty, is a logical choice.
The effect of atorvastatin on the inflammatory markers in the setting of percutaneous coronary angioplasty for unstable angina was predictably favorable. Still other drugs with potential anti-inflammatory activity are investigated for the purpose of further reduction of inflammation and prevention of atherosclerotic plaque rupture, especially in patients with acute coronary syndromes24,25. Cyclooxygenase-2 inhibitors (such as rofecoxib and celecoxib), are a new group of anti-inflammatory agents. Cyclooxygenase-2 is known to be involved in the pathogenesis of inflammatory processes and plaque instability26. It has also been suggested that cyclooxygenase-2 inhibitors could prevent the development of atheroma by interaction with inflammatory mechanisms27. In the present study we demonstrate that the reduction of C-reactive protein and interleukin-6 levels, attained by atorvastatin in a 3- and 6-month observation, is significantly augmented by addition of a cyclooxygenase-2 inhibitor rofecoxib. In the later study by Chenevard etal. another selective COX-2 inhibitor, colecoxib also was effective in reduction of inflammatory markers level. Additionally it improved an endothelial function, assessed by flow-mediated dilatation of the brachial artery, after two weeks of therapy28. However this effect was not confirmed for rofecoxoxib in the Title et al. study, where rofecoxib added to standard therapy (included statin) did not appear to have favorable, additional effect on endothelial dysfunction and inflammation, what could be related to low-risk, highly selected and small group of patients in this trial29.
The hypothesis, that additional anti-inflammatory treatment with COX-2 inhibitors has potential beneficial effect on clinical outcome was recently analyzed in NUT-2 pilot study. In this trial treatment with meloxicam, heparin and aspirin in acute coronary syndromes was associated with significant reduction of adverse events during 90 days follow up comparing to aspirin and heparin group30.
It has to be emphasized, however, that rofecoxib and the related compound celecoxib have recently become a subject of controversy regarding their prothrombotic potential associated with disturbed balance between prostacyclin and thromboxane A2 production. Two recent meta-analyses examined the incidence of cardiovascular thrombotic events in patients receiving either of these drugs. One of these retrospective studies in ca. 18,000 patients suggested that subjects chronically treated with cyclooxygenase-2 inhibitors were at increased risk for myocardial infarction when compared to their counterparts treated with other nonsteroidal anti-inflammatory drugs or to patients treated with aspirin for primary prevention of thromboembolic events31. The other recent analysis of more than 28,000 patients claimed that there was no evidence for an excess of cardiovascular events for rofecoxib relative to either placebo or the non-naproxen nonsteroidal anti-inflammatory drugs that were studied. The differences observed between rofecoxib and naproxen were attributed to the antiplatelet effects of the latter agent32. For the reasons cited above, the administration of cyclooxygenase-2 inhibitors in patients with coronary artery disease may seem controversial. However, their beneficial or harmful actions in patients with coronary artery disease still remain to be precisely elucidated in relation to clinical presentation, specific dose and concomitant medications. In contrast to the majority of trials included in the above meta-analyses, the rofecoxib dose used in the present study was the lowest (12.5 mg/day) of clinically used doses (12.5-50 mg/day) and that all patients received appropriate post- percutaneous coronary angioplasty antiplatelet therapy. None of our 60 patients experienced a thrombotic event during 6 months of observation, however the population of this pilot study is too small to infer about clinical outcomes.
Interleukin-6 concentration has been previously demonstrated to rise in unstable angina and some, but not all studies have shown that its plasma concentration correlates with that of C-reactive protein and with clinical prognosis4. Our study found parallel changes in C-reactive protein and interleukin-6 during the study period.
Limitations of the study
Two principal limitations have to be recognized. First, this study is a pilot investigation with a limited sample size. As such, it only provides a direction for further research to reduce inflammatory activity accompaninig acute coronary syndrome presentation. Considering controversy associated with cycloxygenase 2 inhibitors probably new anti-inflammatory compound should be tested in a randomized, double-blind, clinical, prospective study.
The main goal of this study is to see whether administration of this new drug, if started immediately after an episode of instability and subsequent percutaneous coronary angioplasty, could translate into a decreased incidence of adverse cardiovascular events in patients with unstable angina. The second limitation is the lack of a placebo control group. However, with current knowledge and guidelines, it is extremely difficult to find patients with unstable angina who would not be eligible for lipid-lowering therapy. We therefore chose to deliberately waive the placebo group, even though we realized that this decision may decrease the scientific impact of the study.
Conclusion
In conclusion, on atorvastatin therapy at both tested doses, both C-reactive protein and interleukin-6 levels were reduced at 3 and 6 months. The magnitude of this reduction was further significantly augmented by addition of rofecoxib. Of note, the most profound suppression of C-reactive protein with combined statin and cyclooxygenase-2 inhibitor was found in patients with most pronounced C-reactive protein elevation at baseline. Since these patients are at the highest risk for recurrent cardiovascular events, they may most benefit from the combined anti-inflammatory therapy.

